
BinaxNOW
™
CARD HOME TEST
COVID19 Ag

1 BinaxNOW COVID-19 Ag Card Home Test Instructions for Use
For Use Under an Emergency Use Authorization (EUA) Only
For use with nasal swab specimens
For in vitro Diagnostic Use Only
For Prescription Home Use
INTENDED USE
The BinaxNOW
™
COVID-19 Ag Card Home Test is a lateral flow immunoassay intended for the qualitative detection
of nucleocapsid protein antigen from SARS-CoV-2. This test is authorized for prescription home use with self-collected
observed direct anterior nasal (nares) swab samples from individuals aged 15 years or older who are suspected of COVID-19
by their healthcare provider within the first seven days of symptom onset or adult collected nasal swab samples from
individuals aged four years or older who are suspected of COVID-19 by their healthcare provider within the first seven
days of symptom onset. The BinaxNOW COVID-19 Ag Card Home Test is to be performed only with the supervision of
a telehealth proctor.
The BinaxNOW COVID-19 Ag Card Home Test does not dierentiate between SARS-CoV and SARS-CoV-2.
Results are for the identification of SARS-CoV-2 nucleocapsid protein antigen. Antigen is generally detectable in anterior
nasal (nares) swabs during the acute phase of infection. Positive results indicate the presence of viral antigens, but clinical
correlation with patient history and other diagnostic information is necessary to determine infection status. Positive results
do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of
disease.
Negative results should be treated as presumptive and confirmation with a molecular assay, if necessary, for patient
management, may be performed. Negative results do not rule out SARS-CoV-2 infection and should not be used as the
sole basis for treatment or patient management decisions including infection control decisions. Negative results should
be considered in the context of a patient’s recent exposures, history and the presence of clinical signs and symptoms
consistent with COVID-19.
Individuals who test negative and continue to experience COVID-like symptoms should seek follow up care from their
healthcare provider.
BinaxNOW COVID-19 Ag Card Home Test is only for use under the Food and Drug Administration’s Emergency Use
Authorization.
All prescribing healthcare providers will report all test results they receive from individuals who use the authorized product
to relevant public health authorities in accordance with local, state, and federal requirements using appropriate LOINC and
SNOMED codes, as defined by the Laboratory In Vitro Diagnostics (LIVD) Test Code Mapping for SARS-CoV-2 Tests
provided by CDC.
SUMMARY and EXPLANATION of the TEST
Coronaviruses are a large family of viruses which may cause illness in animals or humans. SARS-CoV-2 is an enveloped,
single-stranded RNA virus of the β genus. The virus can cause mild to severe respiratory illness and has spread globally,
including the United States.
The BinaxNOW COVID-19 Ag Card Home Test is a rapid lateral flow immunoassay for the qualitative detection of
SARS-CoV-2 directly from nasal swabs, without viral transport media. The BinaxNOW COVID-19 Ag Card Home Test kit
contains all components required to carry out an assay for SARS-CoV-2.
PRINCIPLES of the PROCEDURE
The BinaxNOW COVID-19 Ag Card Home Test is an immunochromatographic membrane assay that uses highly sensitive
antibodies to detect SARS-CoV-2 nucleocapsid protein from nasal swab specimens. SARS-CoV-2 specific antibodies and
a control antibody are immobilized onto a membrane support as two distinct lines and combined with other reagents/pads
to construct a test strip. This test strip and a well to hold the swab specimen are mounted on opposite sides of a cardboard,
book-shaped hinged test card.
To perform the test, a nasal swab specimen is collected under observation by or from the patient, then 6 drops of extraction
reagent from a dropper bottle are added to the top hole of the swab well. The patient sample is inserted into the test card
through the bottom hole of the swab well, and firmly pushed upwards until the swab tip is visible through the top hole. The
swab is rotated 3 times clockwise and the card is closed, bringing the extracted sample into contact with the test strip. Test
results are interpreted visually at 15 minutes based on the presence or absence of visually detectable pink/purple colored
lines. Results should not be read after 30 minutes.
REAGENTS and MATERIALS
Materials Provided
Test Cards (1): A cardboard, book-shaped hinged test card containing the test strip
Extraction Reagent (1): Bottle containing <1 mL of extraction reagent
Nasal Swabs (1): Sterile swab for use with BinaxNOW COVID-19 Ag Card Home test
Materials Required but not Provided
Clock, timer or stopwatch
Smart Phone:* Apple is ios11 or newer
Android is version 8 or newer
*Required to download the NAVICA app from the Google play store or Apple app store
COVID19 Ag
BinaxNOW
™
CARD HOME TEST
Healthcare Provider Instructions for Use

2 BinaxNOW COVID-19 Ag Card Home Test Instructions for Use
1
Set Up
It is recommended gloves (not provided) also be used during testing.
DO NOT open items until instructed.
1 Swab 1 Test Card 1 Bottle
B
i
nax
NOW
™
C
O
V
O
O
I
D
-
19
A
g
C
A
RD
S
AMPLE
CON
TR
OL
2
Open Pouch and Scan QR Code on Card
If using a mobile device: If using a computer:
BinaxNOW
™
COVID-19 Ag
CARD
SAMPLE
CONTROL
PRECAUTIONS
1. For in vitro diagnostic use.
2. This test has not been FDA cleared or approved but has been authorized by FDA under an EUA.
3. Federal Law restricts this device to sale by or on the order of a licensed practitioner (US only).
4. This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or
pathogens.
5. This product is only authorized for the duration of the declaration that circumstances exist justifying the authorization
of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of
the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or
authorization is revoked sooner.
6. Proper sample collection and handling are essential for correct results.
7. Leave test card sealed in its foil pouch until just before use. Do not use if pouch is damaged or open.
8. Do not touch swab tip when handling the swab sample.
9. Do not use kit past its expiration date.
10. Do not mix components from dierent kit lots.
11. All kit components are single use items. Do not use with multiple specimens. Do not reuse the used test card.
12. Wash hands thoroughly or use hand sanitizer after handling.
13. Dispose of kit components and patient samples in household trash.
14. INVALID RESULTS can occur when an insucient volume of extraction reagent is added to the test card. To ensure
delivery of adequate volume, hold vial vertically, 1/2 inch above the swab well, and add drops slowly.
STORAGE and STABILITY
Store kit between 35.6-86°F (2-30°C). Ensure all test components are at room temperature before use. The BinaxNOW
COVID-19 Ag Card Home Test is stable until the expiration date marked on the outer packaging and containers.
INITIATING the TELEHEALTH VISIT
Upon receipt of the BinaxNOW COVID-19 Ag Home Test, the patient logs into NAVICA and selects, “I Already Have
a Test Kit”. The home user then visits the telehealth provider website to start testing and waits in queue to connect to the
telehealth proctor.
DIRECTIONS for RUNNING the BinaxNOW
™
COVID-19 Ag CARD HOME TEST
DO NOT OPEN ITEMS UNTIL INSTRUCTED TO DO SO
Wash or sanitize your hands. Make sure they are dry
before starting.
or

3 BinaxNOW COVID-19 Ag Card Home Test Instructions for Use
3
Open Card
Card must stay FLAT on table for entire test.
DO NOT
touch any
parts on
inside of card.
4
Apply Fluid to Top Hole
A. Remove dropper
bottle cap.
B. Hold dropper bottle
straight over TOP HOLE,
not at an angle.
C. Put 6 DROPS into
TOP HOLE. Do not
touch card with tip.
Note: False negative results may occur if less than 6 drops of fluid is used.
5
Open Swab
Keep fingers away from swab end.
A. Open swab package at stick end. B. Take swab out.
6
Swab Left Nostril
A. Insert the entire absorbent tip
of the swab (usually 1/2 to 3/4 of
an inch) into left nostril.
B. Firmly brush against insides of
nostril in a circular motion 5 times
or more for at least 15 seconds.
6 drops
Result Window
Test Strip
Top Hole
Bottom Hole
Up to 3/4 of
an inch.
x5

4 BinaxNOW COVID-19 Ag Card Home Test Instructions for Use
7
Swab Right Nostril
A. Remove swab and insert it
into right nostril.
B. Firmly brush against insides of
nostril in a circular motion 5 times
or more for at least 15 seconds.
Note: False negative results may
occur if the nasal swab is not properly
collected.
8
Insert Swab Into Bottom Hole
Keep card FLAT on table.
Insert swab tip into BOTTOM HOLE and firmly push up until tip fills TOP HOLE.
9
Turn Swab 3 Times
Keep card FLAT on table.
Turn swab to right 3 times in card and leave it in place.
Note: False negative results can occur if the sample swab is not turned prior to closing the card.
10
Peel Strip
DO NOT remove swab.
Keep card FLAT on table.
Keep swab in place. Peel adhesive liner off.
x3
x5

5 BinaxNOW COVID-19 Ag Card Home Test Instructions for Use
11
Close Card and Seal
DO NOT remove swab.
Keep card FLAT on table.
Close left side of card over swab to seal it. Keep card face up on table.
12
Wait 15 Minutes
DO NOT disturb card during this time.
15:00
Note: False results can occur if the card is disturbed/moved or test results are read before 15 minutes.
13
Scan QR Code
If using a mobile device: If using a computer:
BinaxNOW
™
COVID-19 Ag
CARD
SAMPLE
CONTROL
14
Show Result to Your Proctor
BinaxNOW
™
COVID-19 Ag
CARD
SAMPLE
CONTROL
SAMPLE
CONTROL

6 BinaxNOW COVID-19 Ag Card Home Test Instructions for Use
RESULT INTERPRETATION
There are three types of results possible. You will be instructed how to read each type in a specific order. Follow this order
with your proctor:
1. Check for a Positive Result
2. Check for a Negative Result
3. Check for an Invalid Result
Check for Positive COVID-19 Result
Find result window and look carefully for two pink/purple lines in window.
• Positive Result: Two pink/purple lines will appear. One on the top half and one on the bottom half.
COVID-19 was detected.
CONTROL
PositivePositive
Solid Line Faint Line
OR
SAMPLE
Look very closely!
The bottom line can be very
faint. Any pink/purple line
visible here is positive.
Here are photos of actual positive tests. On the right, note how faint the bottom line can get.
A positive test result for COVID-19 indicates that antigens from SARS-CoV-2 were detected, and the patient is
very likely to be infected with the virus and presumed to be contagious. Test results should always be considered in the
context of clinical observations and epidemiological data (such as local prevalence rates and current outbreak/epicenter
locations) in making a final diagnosis and patient management decisions. Patient management should follow current
CDC guidelines.
Check for Negative COVID-19 Result
Find result window and look for a single pink/purple line in window.
• Negative Result: A single pink/purple line on the top half where it says "Control." COVID-19 was not detected.
CONTROL
No Line
Negative
A negative test result for this test means that antigens
from SARS-CoV-2 were not present in the specimen
above the limit of detection. However, a negative result
does not rule out COVID-19 and should not be used
as the sole basis for treatment or patient management
decisions, including infection control decisions. The
amount of antigen in a sample may decrease as the
duration of illness increases. Negative results should be
treated as presumptive and confirmed with a molecular
assay, if necessary, for patient management.
SAMPLE
Check for Invalid Result
If you see any of these, the test is invalid.
CONTROL
No lines seen
Blue control
line only
Pink/purple
sample line only
Blue control line AND
pink/purple sample line
SAMPLE
Dispose In Trash
BinaxNOW
™
COVID-19 Ag
CARD
SAMPLE
CONTROL

7 BinaxNOW COVID-19 Ag Card Home Test Instructions for Use
Reporting Patient Results Using the NAVICA app
Upon completion of the test and result interpretation by the user, the telehealth proctor will send the results to the
user via the NAVICA app and the telehealth provider will report results to relevant public health authorities. The user
will be notified by email and on their mobile device that their results are ready. The user will go to the results screen in
NAVICA to obtain their results.
If the BinaxNOW COVID-19 Ag Card Home Test result is Negative, the user will receive the following:
If the BinaxNOW COVID-19 Ag Card Home Test result is Positive, the user will receive the following:

8 BinaxNOW COVID-19 Ag Card Home Test Instructions for Use
If the BinaxNOW COVID-19 Ag Card Home Test result is Invalid, the user will receive the following:
LIMITATIONS
• This test detects both viable (live) and non-viable, SARS-CoV, and SARS-CoV-2. Test performance depends on the
amount of virus (antigen) in the sample and may or may not correlate with viral culture results performed on the same
sample.
• A negative test result may occur if the level of antigen in a sample is below the detection limit of the test.
• The performance of the BinaxNOW COVID-19 Ag Card Home Test was evaluated using the procedures provided in
this product insert only. Modifications to these procedures may alter the performance of the test.
• False negative results may occur if a specimen is improperly collected or handled.
• False negative results may occur if inadequate extraction buer is used (e.g., <6 drops).
• False negative results may occur if specimen swabs are not twirled within the test card.
• False negative results may occur if swabs are stored in their paper sheath after specimen collection.
• Positive test results do not rule out co-infections with other pathogens.
• False negative results are more likely after eight days or more of symptoms.
• Positive test results do not dierentiate between SARS-CoV and SARS-CoV-2.
• Negative test results are not intended to rule in other non-SARS viral or bacterial infections.
• The presence of mupirocin may interfere with the BinaxNOW COVID-19 Ag test and may cause false negative
results.
• Negative results do not rule out COVID-19 infection and it may be necessary to obtain additional testing with a
molecular assay, if needed for patient management.
• Performance of nasal swabs collected by an adult caregiver from a pediatric patient has not been determined, a study to
support use in a pediatric population is ongoing.
CONDITIONS of AUTHORIZATION for HEALTHCARE PROVIDERS
The BinaxNOW COVID-19 Ag Card Home Test Letter of Authorization, along with the authorized Fact Sheet for
Healthcare Providers, and authorized labeling are available on the FDA website: https://www.fda.gov/medical-devices/
coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/vitro-diagnostics-euas.
However, to assist Healthcare providers using the BinaxNOW COVID-19 Ag Card Home Test, the relevant Conditions of
Authorization are listed below:
A. All prescribing healthcare providers must collect information on the performance of your product in the ordinary
course of business and report to DMD/OHT7-OIR/OPEQ/CDRH (via email: CDRH-EUA-[email protected].
gov) and you (via email: [email protected], or via phone by contacting Abbott Diagnostics Scarborough, Inc.
Technical Service at 1-800-257-9525) any suspected occurrence of false positive or false negative results and
significant deviations from the established performance characteristics of your product of which they become aware.

9 BinaxNOW COVID-19 Ag Card Home Test Instructions for Use
B. All prescribing healthcare providers must report all test results they receive from patients who use your product to
relevant public health authorities in accordance with local, state, and federal requirements, using appropriate LOINC
and SNOMED codes, as defined by the Laboratory In Vitro Diagnostics (LIVD) Test Code Mapping for SARS-CoV-2
Tests provided by the Centers for Disease Control and Prevention (available at: https://www.cdc.gov/csels/dls/sars-cov-
2-livd-codes.html).
PERFORMANCE CHARACTERISTICS
CLINICAL PERFORMANCE
Clinical performance characteristics of BinaxNOW COVID-19 Ag Card Home Test was evaluated in an ongoing multi-site
prospective study in the U.S. A total of four (4) investigational sites throughout the U.S. participated in the study. To be
enrolled in the study, patients had to be presenting at the participating study centers with suspected COVID-19 within 7
days of symptom onset. Each Subject was provided a BinaxNOW COVID-19 Ag Card Home Test. Under the observation
and coaching of a clinical site sta member trained as a proctor, the Subject self-collected one (1) nasal swab and performed
the BinaxNOW COVID-19 Ag Card Home Test. Test results were interpreted and recorded by the Subject or other
home user and independently by the proctor. Parents of pediatric Subjects under the age of 14 or Legally Authorized
Representatives of adult Subjects unable to perform self-collection collected one (1) nasal swab from the Subject,
performed the BinaxNOW COVID-19 Ag Card Home Test, then interpreted and recorded the result for the patient.
An FDA Emergency Use Authorized real-time Polymerase Chain Reaction (RT-PCR) assay for the detection of SARS-
CoV-2 was utilized as the comparator method for this study.
The performance of BinaxNOW COVID-19 Ag Card Home Test was established with 53 nasal swabs collected from
individual symptomatic patients (within 7 days of onset) who were suspected of COVID-19.
BinaxNOW
™
COVID-19 Ag Card Home Test Performance within 7 days of symptom onset against the
Comparator Method
BinaxNOW
™
COVID-19 Ag
Card Home Test
Comparator Method
Positive Negative Total
Positive 22 0 22
Negative
2
28 30
Total
24 28 52*
Positive Agreement: 22/24 91.7% (95% CI: 73.0% - 98.9%)
Negative Agreement: 28/28 100.0% (95% CI: 87.7% - 100.0%)
*1 sample generated an invalid BinaxNOW COVID-19 Ag Card result (0.1% invalid rate)
Performance of BinaxNOW COVID-19 Ag Home Test, with the test performed and results interpreted by the home
user is similar to performance obtained by test operators with no laboratory experience. Due to the relatively small sample
size for the home use clinical study, at the time of the interim analysis, the BinaxNOW COVID-19 Ag Card Home Test
positive agreement established in this ongoing clinical study is estimated to be between 73.0% and 98.9% as reflected
in the 95% Confidence Interval. This is consistent with the performance established in a separate multi-site study in the
US, where the BinaxNOW COVID-19 Ag Card test was performed and results interpreted by test operators with no
laboratory experience. In that study, BinaxNOW COVID-19 Ag Card test positive agreement was 84.6% (95% CI: 76.8%
- 90.6%), refer below:
The performance of BinaxNOW COVID-19 Ag Card was established with 460 nasal swabs collected from individual
symptomatic patients (within 7 days of onset) who were suspected of COVID-19.
BinaxNOW
™
COVID-19 Ag Card Performance within 7 days of symptom onset against the Comparator
Method
BinaxNOW
™
COVID-19 Ag Card
Comparator Method
Positive Negative Total
Positive 99 5 104
Negative 18 338 356
Total 117 343 460
Positive Agreement: 99/117 84.6% (95% CI: 76.8% - 90.6%)
Negative Agreement: 338/343 98.5% (95% CI: 96.6% - 99.5%)
Hazardous Ingredients for the Reagent So
lution
Chemical Name/CAS GHS Code for each Ingredient Concentration
Sodium Azide/26628-22-8 Acute Tox. 2 (Oral), H300
Acute Tox. 1 (Dermal), H310
0.0125%
The solution in the tube contains a hazardous ingredient (see table above). If the solution contacts the skin or eye, flush
with plenty of water. If irritation persists, seek medical advice. http://www.poison.org/contact-us or 1-800-222-1222.

10 BinaxNOW COVID-19 Ag Card Home Test Instructions for Use
Patient demographics, time elapsed since onset of symptoms for all patients enrolled in the above study, are presented in
the table below. Positive results broken down by days since symptom onset:
Days Since
Symptom Onset
Cumulative RT-
PCR Positive (+)
Cumulative BinaxNOW
™
COVID-19 Ag Card Positive (+)
PPA
95 % Confidence
Interval
1 12 10 83.3% 51.6% 97.9%
2 34 28 82.4% 65.5% 93.2%
3 50 41 82.0% 68.6% 91.4%
4 63 50 79.4% 67.3% 88.5%
5 78 63 80.8% 70.3% 88.8%
6 90 75 83.3% 74.0% 90.4%
7 117 99 84.6% 76.8% 90.6%
8 to 10 144 118 81.9% 74.7% 87.9%
11 to 14 161 126 78.3% 71.1% 84.4%
All specimens 167 129 77.2% 70.1% 83.4%
A cohort of patients who presented with symptom onset greater than seven days were enrolled in the clinical study
(n = 161). The positive agreement in patients with symptoms greater than seven days was 60% (30/50) and negative
agreement was 98% (109/111). Therefore, negative results in patients with symptom onset greater than seven days should
be interpreted with caution, as the sensitivity of the assay decreases over time.
ANALYTICAL PERFORMANCE
Limit of Detection (Analytical Sensitivity)
BinaxNOW COVID-19 Ag Card Home Test limit of detection (LOD) was determined by evaluating dierent
concentrations of heat inactivated SARS-CoV-2 virus. Presumed negative natural nasal swab specimens were eluted
in PBS. Swab eluates were combined and mixed thoroughly to create a clinical matrix pool to be used as the diluent.
Inactivated SARS-CoV-2 virus was diluted in this natural nasal swab matrix pool to generate virus dilutions for testing.
Contrived nasal swab samples were prepared by absorbing 20 microliters of each virus dilution onto the swab. The contrived
swab samples were tested according to the test procedure.
The LOD was determined as the lowest virus concentration that was detected ≥ 95% of the time (i.e., concentration at
which at least 19 out of 20 replicates tested positive).
The BinaxNOW COVID-19 Ag Card Home Test LOD in natural nasal swab matrix was confirmed as 140.6 TCID/mL.
Limit of Detection (LoD) Study Results
Concentration
TCID/mL
Number
Positive/Total
% Detected
140.6 20/20 100%
Cross Reactivity (Analytical Specificity) and Microbial Interference
Cross reactivity and potential interference of BinaxNOW COVID-19 Ag Card Home Test was evaluated by testing 37
commensal and pathogenic microorganisms (8 bacteria, 14 viruses, 1 yeast and pooled human nasal wash) that may be
present in the nasal cavity. Each of the organism, viruses, and yeast were tested in triplicate in the absence or presence of
heat inactivated SARS-CoV-2 virus (45 TCID/swab). No cross-reactivity or interference was seen with the following
microorganisms when tested at the concentration presented in the table below.
Potential Cross-Reactant Test Concentration
Virus
Adenovirus
1.0 x 10
⁵
TCID
/mL
Human metapneumovirus (hMPV) 1.0 x 10⁵ TCID/mL
Rhinovirus 1.0 x 10⁵ PFU/mL
Enterovirus/Coxsackievirus B4 1.0 x 10⁵ TCID/mL
Human coronavirus OC43 1.0 x 10⁵ TCID/mL
Human coronavirus 229E 1.0 x 10⁵ TCID/mL
Human coronavirus NL63 1.0 x 10⁵ TCID/mL
Human parainfluenza virus 1 1.0 x 10⁵ TCID/mL
Human parainfluenza virus 2 1.0 x 10⁵ TCID/mL
Human parainfluenza virus 3 1.0 x 10⁵ TCID/mL
Human parainfluenza virus 4 1.0 x 10⁵ TCID/mL
Influenza A 1.0 x 10⁵ TCID/mL
Influenza B 1.0 x 10⁵ TCID/mL
Respiratory Syncytial Virus A 1.0 x 10⁵ PFU/mL

11 BinaxNOW COVID-19 Ag Card Home Test Instructions for Use
Potential Cross-Reactant Test Concentration
Bacteria
Bordetella pertussis 1.0 x 10⁶ cells/mL
Chlamydia pneumoniae 1.0 x 10⁶ IFU/mL
Haemophilus influenzae 1.0 x 10⁶ cells/mL
Legionella pnuemophila 1.0 x 10⁶ cells/mL
Mycoplasma pneumoniae 1.0 x 10⁶ U/mL
Streptococcus pneumoniae 1.0 x 10⁶ cells/mL
Streptococcus pyogenes (group A) 1.0 x 10⁶ cells/mL
Mycobacterium tuberculosis 1.0 x 10⁶ cells/mL
Staphylococcus aureus 1.0 x 10 org/mL
Staphylococcus epidermidis 1.0 x 10 org/mL
Pooled human nasal wash N/A
Yeast Candida albicans 1.0 x 10⁶ cells/mL
To estimate the likelihood of cross-reactivity with SARS-CoV-2 virus in the presence of organisms that were not available
for wet testing, In silico analysis using the Basic Local Alignment Search Tool (BLAST) managed by the National Center for
Biotechnology Information (NCBI) was used to assess the degree of protein sequence homology.
• For P. jirovecii one area of sequence similarity shows 45% homology across 18% of the sequence, making cross-
reactivity in the BinaxNOW COVID-19 Ag Card highly unlikely.
• No protein sequence homology was found between M. tuberculosis, and thus homology-based cross-reactivity can be
ruled out.
• The comparison between SARS-CoV-2 nucleocapsid protein, MERS-CoV and human coronavirus HKU1 revealed that
cross-reactivity cannot be ruled out. Homology for KHU1 and MERS-CoV is relatively low, at 37.8% across 95% of
the sequence and 57.14% across 87% of the sequence, respectively.
High Dose Hook Eect
No high dose hook eect was observed when tested with up to a concentration of 1.6 x 10 TCID/mL of heat inactivated
SARS-CoV-2 virus with the BinaxNOW COVID-19 Ag Card Home Test.
Endogenous Interfering Substances
The following substances, naturally present in respiratory specimens or that may be artificially introduced into the nasal
cavity or nasopharynx, were evaluated with the BinaxNOW COVID-19 Ag Card Home Test at the concentrations listed
below and were found not to aect test performance.
Substance Active Ingredient Concentration
Endogenous
Mucin 2% w/v
Whole Blood 1% v/v
OTC Nasal Drops Phenylephrine 15% v/v
OTC Nasal Gel Sodium Chloride (i.e. NeilMed) 5% v/v
OTC Nasal Spray 1 Cromolyn 15% v/v
OTC Nasal Spray 2 Oxymetazoline 15% v/v
OTC Nasal Spray 3 Fluconazole 5% w/v
Throat Lozenge Benzocaine, Menthol 0.15% w/v
OTC Homeopathic Nasal Spray 1
Galphimia glauca, Sabadilla, Lua
opperculata
20% v/v
OTC Homeopathic Nasal Spray 2 Zincum gluconium (i.e., Zicam) 5% w/v
OTC Homeopathic Nasal Spray 3 Alkalol 10% v/v
OTC Homeopathic Nasal Spray 4 Fluticasone Propionate 5% v/v
Sore Throat Phenol Spray Phenol 15% v/v
Anti-viral Drug Tamiflu (Oseltamivir Phosphate) 0.5% w/v
Antibiotic, Nasal Ointment Mupirocin 0.25% w/v
Antibacterial, Systemic Tobramycin 0.0004% w/v
Testing demonstrated false negative results at concentrations of 5 mg/mL (0.5% w/v). Standard dose of nasal ointment:
2 mg (2% w/w) of mupirocin in single-use 1-gram tubes.
12 BinaxNOW COVID-19 Ag Card Home Test Instructions for Use
Human Factors Study
Abbott conducted a human factor’s study to evaluate whether home user patients or caregivers (lay user) could perform
the test and accurately interpret test results from the BinaxNOW COVID-19 Ag Card under the supervision of a trained
proctor.
In this study, a total of 31 lay users, age 15 and older with either good or corrected vision (far/near-sighted or wear bifocals)
participated in a 45-minute session including an introduction, a product overview, and simulated use cases of BinaxNOW
COVID-19 Ag Card Home test result interpretation. Participants were asked to read and interpret a panel of 9 dierent
BinaxNOW COVID-19 Ag Card test results, including high positive, low positive, negative and invalid under the guidance
of a virtual proctor. Participants and virtual proctors were blinded to the test card results.
22/30 participants described the process of reading and interpreting the test card results as being easy. However, 8/30 of
the participants commented that it was dicult to see some of the fainter line conditions.
A total of 270 trials were recorded in this study. Participants were able to perceive and interpret the results correctly for
239 trials, or 89% of the time. Positive results with stronger intensity lines were easier to read than the positive lines with
less intensity. As the line intensity became fainter, the ability to read the result correctly ranged from 83% to 60%, with an
overall rate of 70%.
After the human factors evaluation, participants were asked for their overall impressions of the instructional materials they
were provided. Nearly all participants (29/30) thought the instructions were straightforward and easy to understand and
follow.
Based on the learnings from this study improvements were made to the Quick Reference Guide and Proctor training.
Usability Study
Abbott conducted a study to evaluate whether a home user can follow instructions from a trained proctor through a
virtual platform and successfully perform the test steps for the BinaxNOW COVID-19 Ag Card test, including nasal swab
collection at home, and correctly interpreting the results.
60 home users, including individuals (n=30) and caregivers (n=30), participated in the study. Each individual or caregiver
pair participated in a 45-minute session with a single proctor. The usability evaluation session included one simulated use
of the BinaxNOW COVID-19 Home Test Kit in which a user was already connected with a proctor, knowledge tasks, and
opportunities to provide feedback.
96.7% (58 out of 60) home users produced a valid result (all negative) and 2 participants produced an invalid result.
(The causes of the invalid tests were insucient amount of reagent added, and damage to the test strip). 58 out of 60
participants interpreted their test result correctly and 2 participants interpreted their result incorrectly (where they
perceived a faint line in the sample window (as positive) when there was none (all results were verified by the study
moderator).
The individual home use group completed 96.8% (1103/1140) of the total tasks/steps correctly. The caregiver home user
group completed 97.3% (1109/1140) of the total tasks/steps correctly. The most common use errors observed during
critical tasks included incorrectly swabbing the nostril to obtain a nasal sample and contacting the test strip with the hands
or with the surface.
90% (56 out of 60) of the home (individual and caregiver) participants had positive impressions of the BinaxNOW
COVID-19 Ag Card Home Test Kit. The test was perceived as being easy to use. The mixed feedback from three home user
participants included that some of the labeling on the dierent components was confusing and one participant reported
that they would not be comfortable performing this test without a medical professional present.
88% (53 out of 60) participants stated the Quick Reference Guide (QRG) shown on the screen while the participant
performed simulated use of the BinaxNOW COVID-19 Ag Card Home test was clear and easy to understand. 54 out of
60 participants felt their proctor that helped guide them through the workflow was helpful and provided clear instructions.
SYMBOLS
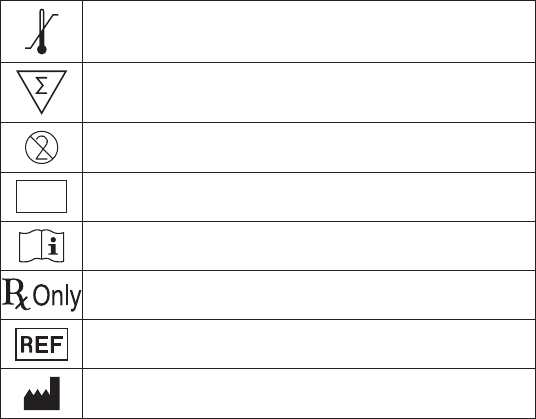
This symbol indicates that the product has a temperature limitation.
This symbol indicates the total number of tests provided in the kit box.
This symbol indicates that the product is for single use only. It is not to be re-used.
IVD
For In Vitro Diagnostic Use.
This symbol indicates that you should consult the instructions for use.
For Prescription Use Only.
This symbol indicates the product’s catalog number.
This symbol indicates the name and location of the product manufacturer.

13 BinaxNOW COVID-19 Ag Card Home Test Instructions for Use
TECHNICAL SUPPORT ADVICE LINE
Further information can be obtained from your Telehealth provider, or by contacting Technical Support on:
US
+ 1 800 257 9525 [email protected]

© 2021 Abbott.All rights reserved.
All trademarks referenced are trademarks of either the Abbott group of companies or their respective owners.
IN195100 Rev.3 2021/03
Abbott Diagnostics Scarborough, Inc.
10 Southgate Road
Scarborough, Maine 04074 USA
www.globalpointofcare.abbott

Abbott
BinaxNOW
COVID-19 Ag Card Home Test
PI - EN
Size:
Flat size: 8.375" x 10.75"
Finished: 8.375" x 5.375"
Printed Colors
PN: IN195100
Rev: 3
CMYK
Incoming Inspection Colors
Date of Last Revision:
3.4 2021/03/28
PMS 2995 U
Primary Blue
PMS 224 U
Magenta-Pink
PMS 303 U
Dark Blue
