
USGS Mineral Resources Program
The Rare-Earth Elements—
Vital to Modern Technologies and Lifestyles
Until recently, the rare-earth elements (REEs) were familiar to a relatively
small number of people, such as chemists, geologists, specialized materials
scientists, and engineers. In the 21st century, the REEs have gained visibility
through many media outlets because (1) the public has recognized the critical,
specialized properties that REEs contribute to modern technology, as well as
(2) China’s dominance in production and supply of the REEs and (3) inter-
national dependence on China for the majority of the world’s REE supply.
Since the late 1990s, China has provided 85 – 95 percent of the world’s
REEs. In 2010, China announced their intention to reduce REE exports. During
this timeframe, REE use increased substantially. REEs are used as components
in high technology devices, including smart phones, digital cameras, computer
hard disks, uorescent and light-emitting-diode (LED) lights, at screen
televisions, computer monitors, and electronic displays. Large quantities of
some REEs are used in clean energy and defense technologies. Because of
the many important uses of REEs, nations dependent on new technologies,
such as Japan, the United States, and members of the European Union, reacted
with great concern to China’s intent to reduce its REE exports. Consequently,
exploration activities intent on discovering economic deposits of REEs and
bringing them into production have increased.
What are the Rare-Earth Elements?
The REE group is composed of 15 elements that range in atomic number
from 57 (lanthanum) to 71 (lutetium) on the periodic table of elements, and are
ofcially referred to as the “lanthanoids,” although they are commonly referred
to as the “lanthanides.” The rare-earth element promethium (atomic number 61)
is not included in discussions of REE deposits because the element is rare and
unstable in nature. Yttrium (atomic number 39) is commonly regarded as an
REE because of its chemical and physical similarities and afnities with the
lanthanoids, and yttrium typically occurs in the same deposits as REEs. Scandium
(atomic number 21) is chemically similar to, and thus sometimes included with, the
REEs, but it does not occur in economic concentrations in the same geological
settings as the lanthanoids and yttrium and will not be discussed further.
Traditionally, the REEs are divided into two groups on the basis of atomic
weight: (1) the light REEs are lanthanum through gadolinium (atomic numbers
57 through 64); and (2) the heavy REEs comprise terbium through lutetium
(atomic numbers 65 through 71). [Note: Some authorities include europium and
gadolinium within the group of heavy REEs.] Yttrium, although light (atomic
number 39), is included with the heavy REE group because of its similar
chemical and physical properties.
Most REEs are not as rare as the group’s name suggests. They were named
“rare-earth elements” because most were identied during the 18th and 19th
centuries as “earths” (originally dened as materials that could not be changed
further by heat) and in comparison to other “earths,” such as lime or magnesia,
they were relatively rare. Cerium is the most abundant REE, and is more common
in the Earth’s crust than copper or lead. All of the REEs, except promethium,
are more abundant on average in the Earth’s crust than silver, gold, or platinum.
However, concentrated and economically minable deposits of REEs are unusual.
As part of a broad mission to conduct research
and provide information on nonfuel mineral
resources, the U.S. Geological Survey (USGS)
supports science to understand the following:
• Where and how concentrations of rare-
earth elements form in the Earth’s crust;
• Where undiscovered/undeveloped resources
of rare-earth elements may occur;
• Trends in the supply and demand of
rare-earth elements domestically and
internationally;
• How undisturbed and mined rare-earth
deposits interact with the environment.
List of the rare-earth elements found in natural
deposits—the “lanthanides” plus yttrium.
[Average abundance (concentration) in the earth’s crust
(in parts per million) from Lide (2004, CRC handbook
of physics and chemistry, 85th edition). For comparison,
average crustal abundances for gold, silver, lead, and
copper are 0.004, 0.075, 14, and 60 parts per million,
respectively]
Element Symbol
Atomic
number
Crustal
abundance
Light REEs
Lanthanum La 57 39
Cerium Ce 58 66.5
Praseodymium Pr 59 9.2
Neodymium Nd 60 41.5
Samarium Sm 62 7.05
Europium Eu 63 2.0
Gadolinium Gd 64 6.2
Heavy REEs
Terbium Tb 65 1.2
Dysprosium Dy 66 5.2
Holmium Ho 67 1.3
Erbium Er 68 3.5
Thulium Tm 69 0.52
Ytterbium Yb 70 3.2
Lutetium Lu 71 0.8
Yttrium Y 39 33
U.S. Department of the Interior
U.S. Geological Survey
Fact Sheet 2014 –3078
November 2014

How Do We Use the Rare-Earth Elements?
Due to their unusual physical and chemical properties,
such as unique magnetic and optical properties, REEs have
diverse applications that touch many aspects of modern life and
culture. Specic REEs are used individually or in combination
to make phosphors—substances that emit luminescence—for
many types of ray tubes and at panel displays, in screens that
range in size from smart phone displays to stadium scoreboards.
Some REEs are used in uorescent and LED lighting. Yttrium,
europium, and terbium phosphors are the red-green-blue
phosphors used in many light bulbs, panels, and televisions.
The glass industry is the largest consumer of REE raw
materials, using them for glass polishing and as additives that
provide color and special optical properties. Lanthanum makes
up as much as 50 percent of digital camera lenses, including
cell phone cameras.
Lanthanum-based catalysts are used to rene petroleum.
Cerium-based catalysts are used in automotive catalytic converters.
Magnets that employ REEs are rapidly growing in
application. Neodymium-iron-boron magnets are the strongest
magnets known, useful when space and weight are limiting
factors. Rare-earth magnets are used in computer hard disks and
CD–ROM and DVD disk drives. The spindle of a disk drive
attains high stability in its spinning motion when driven by a
rare-earth magnet. These magnets are also used in a variety of
conventional automotive subsystems, such as power steering,
electric windows, power seats, and audio speakers.
Nickel-metal hydride batteries are built with lanthanum-
based alloys as anodes. These battery types, when used in
hybrid electric cars, contain signicant amounts of lanthanum,
requiring as much as 10 to 15 kilograms per electric vehicle.
Cerium, lanthanum, neodymium, and praseodymium,
commonly in the form of a mixed oxide known as mischmetal,
are used in steel making to remove impurities and in the
production of special alloys.
The end use applications of REEs are detailed in USGS
Scientic Investigations Report 2011–5094 (available at
http://pubs.usgs.gov/sir/2011/5094/).
Rare-earth elements (REEs) are used in the components of many devices used daily in our modern society, such as: the screens of smart phones,
computers, and flat panel televisions; the motors of computer drives; batteries of hybrid and electric cars; and new generation light bulbs.
Lanthanum-based catalysts are employed in petroleum refining. Large wind turbines use generators that contain strong permanent magnets
composed of neodymium-iron-boron. Photographs used with permission from PHOTOS.com.
Did you know....
Rare-earth magnets are stronger per unit weight
and volume than any other magnet type. Clean energy
technologies, such as large wind turbines and electric
vehicles, use rare-earth permanent magnets (meaning
permanently magnetized) that usually contain four REEs:
praseodymium, neodymium, samarium, and dysprosium.

Where Do Rare-Earth Elements Come From?
The REEs are commonly found together in the Earth’s
crust because they share a trivalent charge (
+3
) and similar ionic
radii. In nature, REEs do not exist individually, like gold or
copper often do, but instead occur in minerals as either minor
or major constit uents. In general, these minerals tend to be
dominated by either light or heavy REEs, although each can
be present. In igneous (magmatic) systems, the large sizes of
the REE ions impede their ability to t into the structure of
common rock-forming minerals. As a result, when common
silicate minerals crystallize — such as feldspars, pyroxenes,
olivine, and amphiboles— most REEs tend to remain in the
coexisting magma. Successive generations of this process
increase REE concentrations in the residual magma until
individual REE minerals crystalize. The REEs can substitute for
one another in crystal structures, and multiple REEs typically
occur within a single mineral.
REEs generally occur in uncommon geologic rock types
and settings. As mentioned earlier, REEs are common in the
Earth’s crust but rarely in economic concentrations. Economic
REE deposits occur primarily in four geologic environments:
carbonatites, alkaline igneous systems, ion-absorption clay
deposits, and monazite-xenotime-bearing placer deposits. Even
within these deposit types, minable (economic) concentrations
of REEs are rare. For example, globally there are more than
500 known carbonatites but only 6 are currently mined for REEs.
Other deposit types can contain minor amounts of REEs
but have not been important REE sources thus far. One example
is the giant Olympic Dam iron oxide-copper-uranium-gold-
silver deposit in Australia, the world’s largest single uranium
deposit, which also contains REE enrichments. So far it has not
proven economical to recover REEs from this deposit.
Carbonatites host the world’s largest REE deposits and
are typically most enriched in the light REEs. Carbonatites are
unusual igneous rocks derived from carbonate-rich magmas, in
contrast to the more common silica-rich magmas. Carbonatites
are igneous rocks with more than 50 percent carbonate minerals,
usually calcite and dolomite. As a group, carbonatites have the
highest REE concentrations of all igneous rocks. Carbonatites
have been the world’s main source for light REEs since the
1960s. Currently, REEs are mined from large carbonatite
bodies in California (Mountain Pass) and in China (Bayan
Obo, Maoniuping, Daluxiang, and Weishan). The Mount Weld
mine in Western Australia, Australia, produces REEs from a
weathered zone that overlies a carbonatite.
Alkaline igneous rocks comprise a group of uncommon
igneous rock types generally decient in silica, relative to
sodium, potassium, and calcium. Many current (2014) advanced
exploration projects are focused on large bodies of alkaline
igneous rocks, with some nding signicant REE concen trations
(0.3 –2.6 percent total REE oxide). These deposit types are sought
because they are often enriched in the important heavy REEs.
Ion-adsorption clay deposits in southern China are the
world’s primary source of heavy REEs. This deposit type is
informally referred to as “south China clays.” Thick clay accu-
mulations that host low concentrations of REEs (from about
0.04 to 0.25 percent total REE oxides) form in tropical regions
with moderate to high rainfall through successive processes:
1. REEs are leached by groundwater from granite bedrock;
2. thick zones of clay-rich soils develop above the granites; and
3. mobilized REEs become weakly xed (by ion-adsorption)
onto clays in the soils.
Despite their low concentrations in REEs, the clay deposits
of south China are economic because the REEs can be easily
extracted from the clays with weak acids, the deposits are often
enriched in high-value heavy REEs, and labor costs are low.
A pilot project in Jamaica is evaluating the recovery
of REEs from tailings of red mud produced by bauxite
(aluminum ore) mining, which could be considered a form
of ion-adsorption clay deposit.
Monazite-xenotime-bearing placer deposits were impor-
tant REE sources prior to the mid-1960s. From some modern
and ancient beach deposits, the REE-thorium-phosphate mineral
monazite [(REEs,Th)PO
4
)] can be recovered as a by-product
during the extraction of the targeted heavy minerals, ilmenite
(FeTiO
3
), rutile (TiO
2
), and zircon (ZrSiO
4
). Ilmenite and
rutile —the principal minerals of value as in these deposits—are
mechanically separated from sand-silt deposits. Monazite can
be recovered simultaneously if desired. The separated ilmenite
and rutile are then chemically processed to remove titanium;
ilmenite and rutile are the primary source of the titanium used
in paint pigments. Monazite is recovered as a byproduct mineral
from beach sands along the southern coasts of India, where it
is sought as a source of light REEs and thorium. The recovered
thorium is stockpiled for future use as fuel material in thorium-
based nuclear power, which is under development. Xenotime
(YPO
4
), a less common mineral, has been recovered as a source
of yttrium and other REEs as a byproduct of mining tin placers.
The mineral monazite is an important source of the REEs.
Monazite, a REE-thorium-phosphate mineral, has been
separated from some ancient and modern beach sands
as a coproduct to the recovery of economic titanium (Ti)
minerals. Photograph courtesy of www.geology.com.
The mineral bastnäsite is
an important source of the
REEs. Bastnäsite, a REE-
carbonate-fluorine mineral,
is the primary ore mineral
in the world’s largest REE
deposits. Photograph
courtesy of Rob Lavinsky,
www.iRocks.com.
Worldwide Supply and Demand for Rare-Earth Elements
As noted earlier, in recent years Chinese production has accounted for
about 95 percent of the REE global market. Citing a need to retain their limited
REE resources for domestic requirements and concerns for environmental effects
of mining, China has restricted the supply of REEs through quotas, licenses, and
taxes. As a result, the REE industry outside of China increased REE stockpiling,
explored for deposits in many locations, and promoted new efforts to conserve,
recycle, and nd substitutes for REEs. New mine production has begun in
Australia (Mount Weld) and the United States (Mountain Pass, California).
In recent years, expert panels convened by research institutes and
government agencies highlighted specic REEs as raw materials critical to
evolving technologies, such as clean-energy applications, high-tech military
components, and electronics (Long and others, 2010). These reports suggest
that a high potential exists for disruptions in REE supplies. As a result,
several expert panel analyses rank REEs high on the “criticality” factor of
raw materials, meaning they are of high technological and economic impor-
tance and have high supply-side risk (National Research Council, 2008). Panels and agencies that assessed the criticality of REEs and
other raw materials include the National Research Council, U.S. Department of Energy, European Commission, American Physical
Society (APS) and Materials Research Society (MRS), and the Resnick Institute.
Worldwide explorations for economic deposits of REEs and efforts to bring them into production have increased substantially
since 2000. More than 400 rare-earth projects were in progress during 2012, including many projects in advanced stages of explora-
tion, meaning that the deposit sizes and REE concentrations were announced based on detailed drilling. One important aspect in the
development of a property for REE mining is the cost and complexity of processing the REE ores. Recovery of REEs can be complex
because they occur in minerals as a group of similar elements, and at many deposits the REEs are hosted within more than one
mineral. Not only do REE-rich minerals need to be concentrated, but the actual elements must be separated from each other, usually
as oxide compounds (for example, lanthanum oxide). The success and timeliness of rare-earth mining projects, and the rare-earth
elements industry in general, is difcult to predict and will be continuously monitored and studied by the USGS.
References
Lide, D.R., ed., 2004, Abundance of elements in the Earth’s crust and
in the sea, in sec. 14 of CRC handbook of physics and chemistry—
A ready-reference book of chemical and physical data (85th edition):
Boca Raton, Fla., CRC Press, p. 17 [table].
Long, K.R., Van Gosen, B.S., Foley, N.K., and Cordier, Daniel,
2010, The principal rare earth elements deposits of the United
States—A summary of domestic deposits and a global perspective:
U.S. Geological Survey Scientic Investigations Report 2010 –5220,
96 p. [Also available at http://pubs.usgs.gov/sir/2010/5220/.]
National Research Council, 2008, Minerals, critical minerals, and the
U.S. economy: Washington, D.C., National Academies Press, 264 p.
[Also available at http://www.nap.edu/openbook.php?record_id=12034.]
For More Information
On production and consumption of rare-earth elements:
http://minerals.usgs.gov/minerals/pubs/commodity/rare_earths/
On historical statistics of rare-earth elements:
http://minerals.usgs.gov/ds/2005/140/
On end use and recycling of rare-earth elements:
http://pubs.usgs.gov/sir/2011/5094/
The USGS Mineral Resources Program is the sole Federal provider
of research and information on rare-earth elements and other nonfuel
mineral resources. For more information, please contact:
Mineral Resources
Program Coordinator Telephone: 703-648-6100
U.S. Geological Survey Fax: 703-648-6057
913 National Center E-mail: [email protected]
Reston, VA 20192 Home page: http://minerals.usgs.gov
Text prepared by Bradley S. Van Gosen, Philip L. Verplanck,
Keith R. Long, Joseph Gambogi, and Robert R. Seal II.
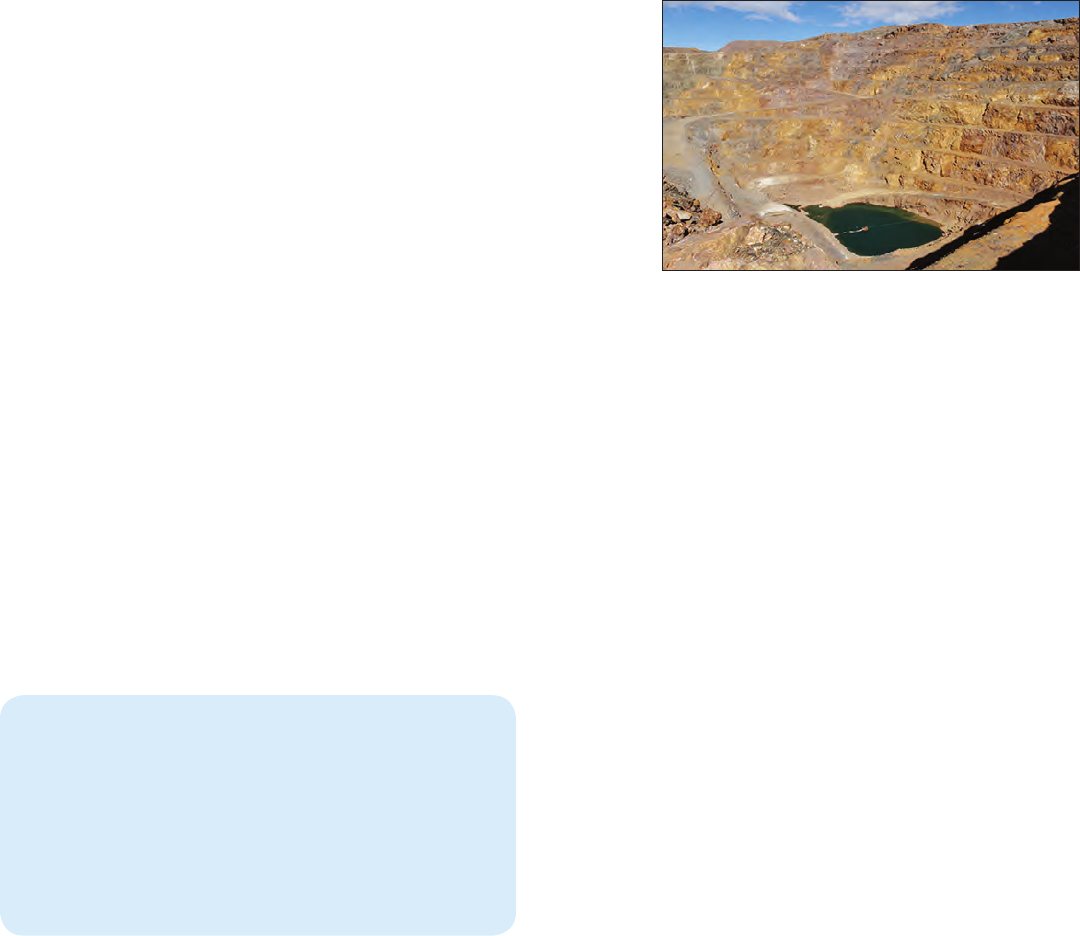
The Mountain Pass mine of Molycorp, Inc., southeastern
California, is the only active producer of REEs in the United
States (2014). The ore body is a carbonatite intrusion,
thought to be the largest REE resource in the United States.
How Do We Ensure Adequate Supplies of
Rare-Earth Elements for the Future?
Global REE resources are estimated to be 110 million
metric tons of rare-earth oxide, which primarily occur, in
descending order, in China, Russia, the United States, India, and
Australia. Exploration for additional REE deposits is ongoing;
therefore, the world’s known in-the-ground resources (endow-
ment) of REEs are likely to increase. Despite many known REE
deposits, the global supply of REEs is limited by the cost and
complexity of exploring REE deposits and developing REE
mines, including REE extraction and separation facilities. These
factors are discussed in USGS Scientic Investigations Report
2010 –5220 (available at http://pubs.usgs.gov/sir/2010/5220/).
In addition to bringing more REE deposits into produc-
tion, other methods may help offset REE supply restrictions.
Examples include new efforts to recycle REEs, research to nd
substitute materials for REEs, and efforts to recover REEs as
coproducts of mineral deposits. These efforts may eventually
offset some of the demand for REEs.
ISSN 2327– 6932 (online)
http://dx.doi.org/10.3133/fs20143078
Did you know....
In the 1940s, as part of the Manhattan Project that
created the nuclear bomb, Frank Spedding and others in the
United States developed chemical ion exchange procedures
that could separate and purify individual REEs. This method
was first used to separate plutonium-239 and neptunium
from uranium, thorium, and actinium in materials generated
by nuclear reactors.
