
U.S. DEPARTMENT OF COMMERCE
National Oceanic and Atmospheric
Administration
National Marine Fisheries Service
Alaska Fisheries Science Center
U.S. DEPARTMENT OF COMMERCE
National Oceanic and Atmospheric
Administration
National Marine Fisheries Service
Alaska Fisheries Science Center
April 2024
NOAA Technical Memorandum NMFS-AFSC-483
2023 Early Spring Collaborative
Pot Sampling (CPS1) for Bristol
Bay District Red King Crab
(Paralithodes camtschaticus)
T. Loher, B. Daly, S. E. Goodman, M. A. Litzow, A. Nault,
E. R. Ryznar, and L. S. Zacher

The National Marine Fisheries Service's Alaska Fisheries Science Center uses the NOAA Technical
Memorandum series to issue informal scientic and technical publications when complete formal
review and editorial processing are not appropriate or feasible. Documents within this series reect
sound professional work and may be referenced in the formal scientic and technical literature.
The NMFS-AFSC Technical Memorandum series of the Alaska Fisheries Science Center continues
the NMFS-F/NWC series established in 1970 by the Northwest Fisheries Center. The NMFS-
NWFSC series is currently used by the Northwest Fisheries Science Center.
This document should be cited as follows:
Loher, T., Daly, B., Goodman, S. E., Litzow, M. A., Nault, A., Ryznar, E. R., and Zacher, L. S. 2024.
2023 early spring collaborative pot sampling (CPS1) for Bristol Bay District red king crab
(Paralithodes camtschaticus). U.S. Department of Commerce, NOAA Technical Memorandum
NMFS-AFSC-483, 66 p.
This document is available online at:
Document available: https://repository.library.noaa.gov
Reference in this document to trade names does not imply endorsement
by the National Marine Fisheries Service, NOAA.

2023 Early Spring Collaborative
Pot Sampling (CPS1) for Bristol
Bay District Red King Crab
(Paralithodes camtschaticus)
T. Loher
1
, B. Daly
2
, S. E. Goodman
1
, M. A. Litzow
3
, A. Nault
2
,
E. R. Ryznar
3
, and L. S. Zacher
3
U.S. DEPARTMENT OF COMMERCE
National Oceanic and Atmospheric Administration
National Marine Fisheries Service
Alaska Fisheries Science Center
NOAA Technical Memorandum NMFS-TM-AFSC-483
April 2024
1
Bering Sea Fisheries Research Foundation
23929 22nd Dr. SE.
Bothell, WA 98021
2
Alaska Department of Fish and Game, Westward Region
Division of Commercial Fisheries
351 Research Court
Kodiak, AK 99615
3
Kodiak Laboratory
Resource Assessment and Conservation Engineering Division
Alaska Fisheries Science Center
National Marine Fisheries Service
National Oceanic and Atmospheric Administration
301 Research Court
Kodiak, AK 99615
ii
iii
ABSTRACT
Between 18 March and 4 April 2023, the Collaborative Pot Sampling (CPS1) project occurred to
collect data on Bristol Bay red king crab (RKC) (Paralithodes camtschaticus). The project
spanned 637 regularly spaced stations placed along 11 transects in Bristol Bay, Alaska. RKC
were fished using modified commercial crab pots (traps) in order to understand early spring
distribution patterns, as well as to collect a suite of biological attributes. A total of 10,191 RKC
were captured at 450 (70.5%) of the survey stations: 76.8% (n = 7,824) of these crabs were male
and 33.2% (n = 2,367) were female. The carapace length (CL) of all RKC was measured and
shell conditions recorded. Females were examined to determine maturity status and the egg
clutches carried by mature females were evaluated for volume and developmental stage. Pop-up
Archival Transmitting tags were deployed on 100 mature-sized male RKC that had recently
molted and were in a new hard-shell condition.
Male RKC ranged from 17.6 to 190.2 mm (0.69 – 7.49 in) CL: 36.1% (n = 2,824) were
immature-sized (< 120 mm (4.7 in) CL); 63.9% (n = 4,999) were mature-sized (≥ 120 mm
(4.7 in) CL); 44.7% (n = 3,497) were legal-sized (≥ 135 mm (5.3 in) CL). Female RKC ranged
from 57.0 to 174.6 mm (2.24 – 6.87 in) CL: 81.7% (n = 1,934) of these individuals were
morphometrically mature. Immature females ranged in size from 57.0 to 99.9 mm (2.24 – 3.93
in) CL and mature females from 82.1 to 174.6 mm (3.23 – 6.87 in) mm CL. No molting males
and only five molting females were captured, the latter of which were all immature. No barren
mature females were encountered. Overall, 43.5% of mature females carried egg clutches that
were approximately three-quarters full and 53.0% carried full clutches.
Spatial distribution of mature-sized males and females did not depart markedly from those
observed during the subsequent (2023) National Marine Fisheries Service trawl survey.
However, for immature crabs of both sexes, the distributions from CPS1 were more similar to
the 2023 trawl survey than to the 2022 trawl survey. For crabs of mature and legal sizes,
distributions observed during CPS1 were less patchy than suggested by the summer trawl survey
data. Across all demographics, the majority of crab were caught within the Nearshore Bristol
Bay Trawl Closure Area (NBBTCA): 66% of all RKC were captured in the NBBTCA and
percentages by demographic ranged from a low of 61.7% for legal-sized males to a high of

iv
75.8% for mature females. For males, just under 20% of individuals were encountered in the Red
King Crab Savings Area (RKCSA). For females, the proportion of crab captured inside the
RKCSA was 17.4% for mature individuals versus 7.6% for immature crabs.
In addition to RKC, 570 Tanner crabs (Chionoecetes bairdi) were captured at 227 stations; 2,393
yellowfin sole (Limanda aspera) were captured at 420 stations; and 1,728 Pacific cod (Gadus
macrocephalus) were captured at 510 stations. Smaller numbers of snow crab (Chionoecetes
opilio), Tanner-snow crab hybrids, horsehair crab (Erimacrus isenbeckii), and Pacific lyre crab
(Hyas lyratus) were encountered, in addition to great sculpin (Myoxocephalus
polyacanthocephalus), walleye pollock (Gadus chalcogrammus), Pacific halibut (Hippoglossus
stenolepis), northern rock sole (Lepidopsetta polyxystra), starry flounder (Platichthys stellatus),
Alaska plaice (Pleuronectes quadrituberculatus), Alaska skate (Bathraja parmifera),
unidentified skates (Rajidae), and sunflowers seastars (Pycnopodia helianthoides). Tags were
deployed on male RKC for 57 – 75 days and had a 91% success rate in providing reliable
locations with minimal error ellipses. There was a high degree of variability in direction of
movement among individuals, but movement rates averaged 0.83 ± 0.50 km/day (0.45 ±
0.27 nmi/day), with a range of 0.08 to 2.53 km/day (0.04 to 1.37 nmi/day), and a prevailing
overall trend of movement to the north and northeast.
v
CONTENTS
ABSTRACT ............................................................................................................................... iii
INTRODUCTION ..................................................................................................................... 1
METHODS ................................................................................................................................ 6
Survey Design and Pots ....................................................................................................... 6
Oceanographic Sampling ..................................................................................................... 9
Red King Crab Sampling ..................................................................................................... 9
Bycatch Sampling ................................................................................................................ 12
Red King Crab Tagging ....................................................................................................... 12
Graphical and Statistical Analyses....................................................................................... 13
Public Data Repository ........................................................................................................ 14
RESULTS .................................................................................................................................. 14
Survey Completion .............................................................................................................. 14
Oceanographic Conditions ................................................................................................... 15
Red King Crab Catch Composition ..................................................................................... 15
Red King Crab Spatial Distribution ..................................................................................... 17
Other Crab Species .............................................................................................................. 18
Fish and Macroinvertebrates ................................................................................................ 19
Red King Crab Tagging ....................................................................................................... 19
DISCUSSION ............................................................................................................................ 20
ACKNOWLEDGMENTS ......................................................................................................... 25
CITATIONS .............................................................................................................................. 27
vi
TABLES .................................................................................................................................... 36
FIGURES ................................................................................................................................... 38
1
INTRODUCTION
Red king crab (Paralithodes camtschaticus: hereafter “RKC”) once represented one of the most
lucrative fisheries in the Bering Sea and Gulf of Alaska. Following rapid capitalization, the
Bristol Bay fishery exploded in the late 1970s, supported by a mature male biomass that peaked
at ~130,000 metric tons (t) (Zacher et al. 2024). However, in the early 1980s, Bristol Bay RKC
(BBRKC) productivity collapsed by an order of magnitude, culminating in closure of the
directed fishery in 1983. Fishing resumed the following year, but it exploited a population that
was composed of a considerably lower biomass of both mature-sized males (i.e., ≥ 120 mm
(4.7 in) carapace length (CL)) and reproductively mature females (Fig. 1). Since the population
collapse of the early 1980s, stock abundance has been relatively low and variable, with the
abundance of legally harvestable males (i.e., ≥ 135 mm (5.3 in) CL or ≥ 165 mm (6.5 in)
carapace width (CW)) fluctuating between a low of approximately 5,400 t in 1985 and a high of
~52,000 t in 1991 (Zacher et al. 2024). The 1991 peak had appeared to represent a period of
stock recovery, but the fishery was closed for a second time in 1994 due to declining female
spawning biomass and remained closed in 1995. This second closure period led to the adoption
of a rebuilding plan, supported by the development of a length-based stock assessment model
(Zheng et al. 1995a, 1995b) and a formal management strategy evaluation (Zheng et al. 1997a,
b). A modest increase in RKC abundance followed and continued through the early 2000s.
However, a declining trend in total biomass has been observed in recent years for both mature
males and females, with the decline in the males beginning in approximately 2004 and since at
least 2014 for females (Fig. 1). The 2021 female spawning stock biomass is estimated to have
been at its lowest level since 1995 (Zacher et al. 2024) and the fishery was once again closed for
the 2021/22 fishing season; it remained closed for the 2022/23 season, since estimated mature
female biomass increased by less than 4% relative to 2021 (Zacher et al. 2024). In response to
the consecutive closures of the BBRKC fishery in 2021 and 2022, in September 2022 the Alaska
Bering Sea Crabbers (ABSC), a trade organization representing Bering Sea crab harvesters,
submitted a petition to the North Pacific Fishery Management Council (NPFMC; the “Council”)
requesting short-term emergency action to protect RKC and crab habitat from fisheries-induced
disturbances. Management of commercial fisheries in the Bristol Bay District has a long history
of restrictive measures that have resulted in the establishment of a series of closure areas in
2
which the activities of various harvest sectors are limited. Four closure areas (Fig. 2) are of
particular relevance to the management of BBRKC:
1) Bycatch Limitation Zone 1 (BLZ1), encompassing Bristol Bay waters north of the
Alaska Peninsula, south of 58° 00’ N lat., and east of 165° 00’ W long.; this is further
divided into Reporting Area 516 spanning 162° 00’ to 163° 00’ W long. and Reporting
Area 509 spanning 163° 00’ to 165° 00’ W long.
2) The Red King Crab Savings Area (RKCSA), spanning 56° 00’ to 57° 00’ N lat. and
162° 00’ to 164° 00’ W long.
3) The Red King Crab Savings Subarea (RKCSS), defined as waters within the RKCSA
between 56° 00’ and 56° 10’ N lat.
4) The Nearshore Bristol Bay Trawl Closure Area (NBBTCA), defined as all Bristol Bay
waters east of 162° W long., within which lies the Northern Bristol Bay Trawl Area
(NBBTA), located to the south and west of the Nushagak Peninsula, spanning 58° 00’ to
58° 43’ N lat. and 159° 00’ to 160° 00’ W long.
BLZ1 was the first of these management areas to be established, via Amendment 10 to the
Bering Sea and Aleutian Islands (BSAI) Groundfish Fishery Management Plan (FMP).
Amendment 10 was first enacted via emergency rule on 3 June 1986 (U.S. Department of
Commerce 1986) and permanently adopted in March of 1987 (U.S. Department of Commerce
1987). The Amendment addressed concerns that bycatch of RKC and Tanner crabs
(Chionoecetes bairdi) by domestic and joint-venture groundfish trawl fisheries was contributing
to low crab abundance. Amendment 10 closed a section of BLZ1 (waters between 160° 00’ W
and 162° 00’ W long. from the Alaska Peninsula in the south to 58° 00’ N lat.) to trawling year-
round. Areas immediately to the east and west within BLZ1 remained open to trawl fisheries but
with bycatch limits for RKC and Tanner crabs established for both domestic and foreign fleets
targeting yellowfin sole (Limanda aspera) and other flatfish. Amendment 12A, enacted in 1989
(U.S. Department of Commerce 1989), established Statistical Area 516 spanning 162° 00’ to
163° 00’ W long. and closed that area to all trawl gear between 15 March and 15 June each year,
with an exception for directed fishing for Pacific cod (Gadus macrocephalus) along the Alaska
Peninsula, south of a line running from northeast to southwest and approximating waters
3
shallower than 25 fm, subject to a bycatch cap of 12,000 RKC. Between 160° 00’ and
162° 00’ W long., the Amendment allowed the U.S. Secretary of Commerce to authorize trawl
fishing for Pacific cod “provided that such fishing is in compliance with a scientific data
collection and monitoring program, established by the Regional Director after consultation with
the Council”. Currently, Area 516 of BLZ1 is closed to all trawling from 15 March through
15 June (U.S. National Archives and Records Administration 2023).
The RKCSA was first established by emergency interim rule in January 1995, in response to the
RKC fishery closures of 1994-95. It closed the area to all trawl gear from 20 January through
25 April 1995, “to conserve the female red king crab stocks in the Bristol Bay area” (50 CFR
Parts 675 and 677; U.S. Department of Commerce 1995a). That December, the closure was
formalized as an inseason adjustment spanning 20 January through 31 March 1996, with the
stated objective of preventing “an excessive share of red king crab from being taken by the
groundfish trawl fisheries early in the fishing season” (50 CFR Part 675; U.S. Department of
Commerce 1995b). The December action redefined the closure as applying only to “directed
fishing for groundfish by vessels using other than pelagic trawl gear”. In January 1996 the
RKCSA became a permanent year-round closure for non-pelagic trawling (50 CFR Part 679;
U.S. Department of Commerce 1996) and included the establishment of the RKCSS. In
consideration of the area’s importance to rock sole (Lepidopsetta polyxystra) fisheries, the
RKCSS was to “remain open to nonpelagic trawling for groundfish during the years in which a
guideline harvest level for Bristol Bay red king crab is established.” Within this area, a separate
prohibited species catch (PSC) limit was established that was constrained to no more than 35%
of the total PSC apportioned to the rock sole fishery in any given year. The same ruling also
established the Nearshore Bristol Bay Trawl Closure Area (NBBTCA), in which all trawling was
prohibited except within the Northern Bristol Bay Trawl Area (NBBTA): in the NBBTA,
trawling was allowed from 1200 hr on 1 April through 1200 hr on 15 June of each year (U.S.
Department of Commerce 1996). Although not explicitly stated as justification within the ruling,
the decision to allow trawling within the NBBTA appears to have been in response to written
public commentary that suggested that “Support exists for … leaving open the subarea between
58° and 58°43’ N. lat., which is a productive yellowfin sole fishing ground”, and to which NMFS
indicated agreement. ABSC’s 2022 petition requested that the RKCSA and RKCSS be closed to

4
all fishing gears from 1 January to 30 June 2023, by emergency rule. The NPFMC declined to
enact the proposed rule. However, it passed a motion (i.e., D2 Council Motion BBRKC) at its
October 2022 meeting that encouraged the development of “methods to gather data on
interannual and seasonal distribution of crab, such as additional surveys and tagging studies”.
A prior discussion paper, prepared for the Council’s April 2022 meeting, Cunningham and Cates
(2022) had sought to provide “information on Bristol Bay red king crab molting/mating annual
cycle and how seasonality of this overlap with fisheries” and to provide guidance regarding
“responsive spatial management measures … and how they might be applied to protect BBRKC.”
A subsequent analysis responding directly to the ABSC’s emergency rule request (Cates et al.
2022) identified a lack of objective, quantitative information on RKC distributions that could be
used to guide bycatch avoidance throughout the year. In particular, the most unbiased
information on RKC distributions is obtained from the NMFS trawl survey; however, such data
represent only snapshots of summer distribution. Data obtained from the directed fishery provide
insight into the seasonal redistribution of legal-size males (Zacher et al. 2018); however, these
data fail to represent other demographics (i.e., sex, size, or maturity categories) of interest, such
as female spawning stock and sublegal-size individuals of both sexes. Additional information
regarding seasonal redistribution and migration patterns is needed.
Seasonal migration is a common feature of commercially exploited marine species that reside in
the Bering Sea and Aleutian Islands region, including walleye pollock (Gadus chalcogrammus;
Kotwicki et al. 2005), Pacific cod (Bryan et al. 2021), Pacific halibut (Hippoglossus stenolepis;
Loher 2022), and snow crab (Chionoecetes opilio; Nichol and Somerton 2015). For RKC,
seasonal migration of reproductive females has been documented in southeast Alaska using
acoustic tags (Stone et al. 1992). Analyses of fishery logbook and catch data (Zacher et al. 2018)
have suggested that legal-size male crabs in Bristol Bay are found farther to the south and west
in autumn than during the summer, and/or farther from the Alaska Peninsula. These data also
demonstrate that the relative proportion of legal-size males taken from trawl-exclusion areas
varies interannually, likely as a function of temperature. Sex-specific differences in migratory
behavior may arise from differences in life history between the sexes. For example, egg-bearing
females may be more limited in their abilities to move because they need to remain at
temperatures that allow for successful embryo development. In contrast, males may be able to

5
redistribute throughout the year in order to find habitat that represents a metabolic thermal
optimum for feeding and growth (sensu Hernández-Sandoval et al. 2018). Regardless of the
drivers that are involved, RKC distribution within Bristol Bay is not likely to be static throughout
the year and summer survey data may be insufficient to provide year-round guidance for
avoidance of RKC bycatch at optimal spatial and temporal resolutions.
In addition to obtaining survey data outside of the summer season, Pop-up Archival Transmitting
(PAT) tags can be a powerful tool for studying the movement and environmental conditions
experienced by animals in cases in which data that span specific periods of time are desired. PAT
tags are electronic tags that contain a sensor package, an automated release mechanism, and
satellite-broadcast capabilities, allowing for environmental data to be collected while attached to
the host animal and recovered via satellite telemetry. Broadcast dates (and, hence, period at
liberty) may be pre-specified, and the tag’s final position is determined by the receiving
satellite’s use of the Doppler shift in the received signal (Keating 1995). This has the advantage
of allowing for determination of final location even if individuals move to areas absent of fishery
effort or where reporting of physical recoveries is reduced (e.g., in Russian waters for animals
tagged in Alaska). Additionally, locations during time at liberty can, under ideal conditions, be
inferred from archived light data (sensu Block et al. 1998, Loher 2022) and may allow for state-
space modelling of movement (Pederson et al. 2018, Nielsen et al. 2019) that cannot be achieved
using conventional mark-recapture data. Satellite-tagging studies of BBRKC were first initiated
in July 2020 to study intra-annual movement patterns and the seasonal use of trawl closure areas.
Initial work focused on summer to fall movement of mature-size males to compare data derived
from the fall directed fishery to tagging. More recently, efforts have focused on elucidating
movement into winter and spring, which are particularly important seasons for red king crab,
encompassing molting, larval release, and mating, as well as being a period of higher bycatch in
trawl fisheries. From 2020 through 2022, 470 satellite tags have been deployed on eastern Bering
Sea red king crab (225 females, 245 males) through collaborate research between NMFS, the
Alaska Department of Fish and Game (ADF&G), and the Bering Sea Fisheries Research
Foundation (BSFRF). However, our understanding of the seasonal distribution of BBRKC males
and females in the winter/spring is still incomplete. Using satellite tags on males and primiparous
(i.e., first-spawning) females is challenging because tag attachment does not allow tags to be

6
retained through the molt and these crab molt in late winter (~February). Multiparous females
(i.e., individuals that have spawned previously) molt later in the spring and, thus, tags can be left
on these animals for longer periods; however, scaling individual movement vectors to population
level distribution patterns is challenging.
In the current study, a sampling project employing modified commercial king crab pots (traps)
was executed during early spring, 2023, within a portion of the Bristol Bay RKC management
district to: a) evaluate crab distribution in comparison to those observed during summer trawl
surveys; b) investigate maturity, molting, and reproductive status, and; c) tag mature-size male
red king crabs with satellite-transmitting archival tags programmed to detach and report during
the summer of 2023, coincident with the prosecution of the summer trawl survey.
METHODS
Survey Design and Pots
The complete survey design was composed of a total of 694 stations arranged in 11 transects,
each running approximately north-to-south, with transects separated by 15.0 nautical miles (nmi)
(27.7 km) and spanning approximately 159° 39’ to 164° 16’ W long. from east to west (Fig. 3).
Individual stations were positioned 2 nmi (3.7 km) apart along each transect and the transects
extended from shallow waters (i.e., minimum depths of 14 fm (26 m)) in southern Bristol Bay to
approximately 57° 50’ N lat. This design resulted in a grid that covered approximately 35% of
the spatial extent of the BBRKC management District and falling within an area that during the
NMFS EBS Continental Shelf summer trawl survey (Zacher et al. 2024) is represented by 47
trawl stations. Over the last 10 years, this area has contained, on average, 91 ± 1 % of all mature
female and 83 ± 2 % of all mature-sized male RKC that have been captured during NMFS EBS
summer trawl surveys.
Fishing was conducted by two vessels (Fig. 4):
1) FV Silver Spray, a 130-ton (118 t), house-forward crab vessel measuring 116 ft
(35.4 m) in length overall (LOA) and 30 ft (9.1 m) beam; homeport Kodiak, AK.
7
2) FV Summer Bay, a 196-ton (178 t), house-aft crab vessel measuring 107 ft (32.6 m)
LOA and 26 ft (7.9 m) beam; homeport Dutch Harbor, AK.
At each station that was fished during the pot survey, latitude and longitude of pot-setting was
recorded to the nearest 0.01 ft as determined by the GPS unit on the vessel’s bridge and recorded
at the vessel’s position when the pot first landed in the water. Water depth at the same position,
according to the depth sounder on the bridge, was recorded to the nearest 0.1 fm (~ 0.2 m) and
time set and hauled were recorded, to the nearest minute, as the time that the pot left the pot
launcher until hauling began, defined as the time when line retrieval via the pot hauler was
initiated.
Fishing was conducted using modified square commercial king crab pots. The pots used on both
vessels were constructed of a double frame: an outer frame composed of 1.5 in (38 mm) round
steel rod; and an inner frame, which supported the pot’s webbing, composed of 0.75 in (19 mm)
round steel rod (Fig. 5). On the Silver Spray, the outer frame measured 80.5 in (2.05 m) square
by 33 in (0.84 m) tall and the inner frame was 77.25 in (1.96 m) square by 31 in (0.79 m) tall; on
the Summer Bay, the outer frame measured 84 in (2.1 m) square by 34 (0.86 m) tall and the inner
frame was 78 in (2.0 m) square by 32 in (0.81 m) tall. The double-frame design of these pots
allows for the outer frame to take the landing force and abrasion of the seabed while protecting
the pot’s webbing from damage. All pots contained two rectangular funnels on opposing sides of
the pot. The funnels spanned the entire width and height of the pot wall at their outer opening
and tapered to a rectangular opening inside of the pot. Each funnel was upward-sloping (i.e., the
floor of the funnel was longer than its roof) and the opening was mounted in the funnel’s roof
such that the funnel’s opening pointed largely upwards (Fig. 5, upper). On the Summer Bay, the
funnel openings measured 38 in (96.5 cm) by 11 in (28.0 mm) and extended a maximum distance
of ~28 in (70 cm) into the pot at the lower lip of the opening, which was positioned
approximately 22 in (56 cm) above the floor of the pot (i.e., this is the distance that a crab would
“drop” upon entering an empty pot) (Fig. 5). The funnels of the pots used by the Silver Spray
were of slightly variable specifications: funnel width was 34.5 in (87.6 cm) with heights ranging
from 7.5 in to 9 in (19.0 to 22.0 mm); the funnels extended a maximum distance of 23.25 in
(59.0 cm) into the pot at the lower lip of the funnel’s opening and were positioned between 16 in
8
and 20 in (40.6 to 50.8 cm) above the floor of the pot. Funnels on the pots used by the Summer
Bay were not fitted with triggers or funnels. Some pots used by the Silver Spray were fitted with
plastic funnel hoods; however, these hoods were propped open (Fig. 5) in order to disable them
and not impede the entry of crabs into the pot (Fig. 5). On pots used aboard the Silver Spray, the
body was lined with 5.25 in (13.3 cm) stretched-mesh webbing and the funnels lined with 3.75 in
(9.5 cm) stretched-mesh webbing. On pots used aboard the Summer Bay, the body was also lined
with 5.25 in (13.3 cm) stretched-mesh webbing and the funnels lined with 3.5 in (8.9 cm)
stretched-mesh webbing. These webbing sizes are smaller than used on red king crab pots during
commercial fishing, which are required to contain at least one panel composed of a minimum of
9 in (22.9 cm) stretched-mesh. Smaller meshes were employed herein to enhance the retention of
sublegal-size and juvenile crabs. Where pots had been constructed with escape rings, these were
covered by webbing and therefore not functional; again, this was to prevent small crabs from
escaping the pots. All pots were equipped with a section of webbing that was secured with
biodegradable cotton twine, which would degrade and allow crabs and other bycatch to escape
from the pots if they were lost on the grounds. Pots weighed between 760 and 810 pounds (345-
367 kg), unbaited.
Each pot was baited using approximately 8 pounds (3.6 kg) of fresh-frozen (i.e., unsalted),
chopped Pacific herring (Clupea pallasii) that was contained within a single 14 in × 18 in (36 cm
× 46 cm) bait bag hung from the center of the pot’s roof (Fig. 5, upper) and introduced into the
pot through a side-panel that also served as the door for emptying the pot of its catch (Fig. 5,
lower). Each pot contained a bridle that was attached to two “shots” (i.e., 33 fm (60 m)) of buoy
line: one shot of sinking (nylon) line was attached to the pot bridle and one shot of floating
(polypropylene) line led from the sinking line to the first pot buoy. Two buoys were employed:
an A3 “diver” buoy (i.e., measuring approximately 17 in (43 cm) in diameter and 23 in (58 cm)
long, including the eye, and containing ~14.5 gal (55 l) of air); followed by a shorter length (~10
fm; 18 m) of floating line that led to an LD2 (11 in (28 cm) by 24 in (61 cm)) trailer buoy. In
addition, the Silver Spray employed a standard 12 in (15 cm) by 6 in (30 cm) cork as a third and
final buoy. When fishing particularly shallow stations, the Silver Spray removed one shot of
buoy line.
9
Oceanographic Sampling
Oceanographic conditions and depths fished were monitored using dataloggers affixed to the
pots. A combination of three models of datalogger manufactured by RBR Ltd. (Ottawa, Ontario,
Canada; https://rbr-global.com/about-rbr/contact-rbr/) were employed: 1) XR-420-CTD (n = 11);
2) RBRduet TD (n = 6), and; 3) TDR-2050 (n = 4). The XR-420-CTD is a cylindrical unit that
measures 310 mm (12.2 in) in length and 64 mm (2.5 in) in diameter and records temperatures
between -5° and 35° C (23° and 95° F) at a resolution of < 0.00005° C (< 0.00009° F) and
nominal accuracy of ± 0.002° C (0.0036° F); and conductivities (i.e., salinities) from 0 to 85
mS/cm at 0.01 resolution and nominal accuracy of ±0.003 mS/cm at 35 psu and 15° C (59° F).
The RBRduet TD is a cylindrical unit that measures 200 mm (7.9 in) in length and 25.4 mm (1.0
in) in diameter and records temperature at the same resolution as the XR-420-CTD. TDR-2050s
were the manufacturer’s precursor to the RBRduet, measured 235 mm (9.3 in) by 38 mm (15.2
in) and recorded temperature at resolutions equivalent to the RBRduet. The majority of loggers
(n = 19) were equipped with pressure transducers rated to 2,000 decibar (where 1 dbar of
pressure is nearly equivalent to 1 m (3.28 ft) of seawater); each vessel carried a single logger
with a 1,000-dbar transducer. These transducers were capable of determining water depth to a
resolution of < 2 m (< 6.56 ft) at a nominal accuracy of 1 m (3.28 ft); and a resolution of < 1 m
(< 3.28 ft) at a nominal accuracy of 0.5 m (1.64 ft), respectively. All loggers were owned and
administered by ADF&G who routinely return the units to RBR for calibration every three years,
on a rotating basis. For deployment, loggers were placed inside the crab pots, attached to their
roof (Fig. 6), and programmed to record each measured parameter at 10-minute intervals
throughout the pot soak.
Red King Crab Sampling
All RKC that were captured were processed and biological data collected from each crab.
Subsampling protocols were developed in case larger numbers of crabs than could reasonably be
processed were captured at any individual station; however, these protocols did not need to be
employed. The carapace length (CL) of each RKC was measured to the nearest 0.1 mm as the
distance across the crab’s dorsal surface from the center of the posterior margin of the carapace
10
to the base of one of the eye orbits at the base of the rostrum (Fig. 7, upper). Measurements were
taken using a variety of mechanical and digital calipers (Fig. 7, lower): 1) Mitutoyo Research
and Development (Kirkland, Washington, USA) mechanical Vernier calipers; 2) Sylvac (Fowler
High Precision, Canton, Massachusetts, USA) S Cal Pro digital calipers, and; 3) iGaging (San
Clemente, California, USA) Absolute Origin IP54 0-12 in bluetooth-enabled digital electronic
calipers.
RKC were identified by sex and additional data were collected depending upon the sex of the
individual. Shell condition in female RKC may be an indicator of imminent breeding potential,
as female RKC do not store sperm (i.e., unlike Tanner and snow crabs (Chionoecetes opilio);
Adams and Paul 1983, Saint-Marie and Lovrich 1994) and must therefore mate during each
year’s molting cycle. Shell hardness may affect a crab’s vulnerability to fishing gear and
subsequent probability of survival following return to the sea (sensu Stoner et al. 2008, Yochum
et al. 2017), and shell condition in both sexes is used as an input in stock assessment models as it
may be a predictor of parameters such as molting probability, growth increment, and mortality
rates (Zheng et al. 2021). For all crabs, shell condition was assigned to one of the following
categories, consistent with the descriptions found in Donaldson & Beyersdorfer (2003): 0 =
premolt or molting; 1 = recently-molted, soft and pliable; 2 = new hard-shell, both firm and
clean; 3 = old-shell, slightly worn; 4 = old-shell, worn; 5 = very old-shell. Note that shell
hardening in RKC is a progressive process in which the shell can require in excess of 2 months
post-molt to achieve full harness (Stevens 2009). Evaluation of whether any given shell was
“pliable” during the current survey was determined subjectively; that is, no quantitative measures
of shell “hardness” (e.g., via durometer readings: Stevens 2009) were used to determine the
division between Shell Conditions 1 and 2.
For mature female RKC, a given individual’s reproductive status and position in the molt-mate
cycle can be inferred from an examination of the eggs that she bears. Completion of the molt-
mate cycle is indicated by uneyed (i.e., recently extruded) eggs. Conversely, the presence of eyed
eggs (i.e., containing visible embryos), hatching eggs, or empty egg cases indicate the
progression of egg incubation toward and through larval release; and absence of eggs in
morphologically-mature females (a.k.a. “barren”) may be an indicator of reproductive failure
11
(e.g., environmentally or physiologically induced (Ganji 2011) skipped spawning (sensu
Jørgensen et al. 2006), or a relative lack of suitable mates (sensu Baker et al. 2022)). For female
RKC, egg clutch assessments were conducted as per NMFS EBS trawl survey protocols (Zacher
et al. 2024). The presence or absence of eggs was noted and the development stage (i.e., egg
“condition”) and the size of each egg clutch was recorded. Egg condition categories were defined
as follows: 0 = no eggs; 1 = uneyed; 2 = eyed; 3 = dead; 4 = empty egg cases; 5 = hatching.
Determination of the presence/absence of “eyes” (i.e., developing embryos) within the eggs was
conducted with the naked eye and did not employ magnifying devices. Clutch size categories
were defined relative to the expected size of a full clutch, where a full clutch is expected to fully
cover the abdomen and causes considerable distension of the abdominal flap due to its mass
(Donaldson and Beyersdorfer 2003). Clutch fullness was assigned to the following categories:
0 = no eggs, crab is immature; 1 = mature crab with no eggs; 2 = trace to 1/8 clutch; 3 = 1/4
clutch; 4 = 1/2 clutch; 5 = 3/4 clutch; 6 = full. Assignment to a clutch fullness category was
subjective (i.e., no empirical volumetric measurements were taken) and each crab was assigned
to the fullness category that came closest to its estimated clutch size (e.g., a female whose clutch
appeared to be 60% full would be assigned category “4” because it is closer to 50% than to
75%). For female RKC that were not egg-bearing, maturity was determined on the basis of
ventral anatomy, according to Donaldson and Beyersdorfer (2014). In mature females, the
abdominal flap entirely covers the first coxa (i.e., basal section) of each of the walking legs,
whereas in immature individuals the abdominal flap is relatively small and the coxae of the
walking legs are exposed.
For male lithodid crabs, morphometric maturity may be inferred from changes in chela (claw)
height relative to carapace length and width (sensu Olsen 2016) and physiological maturity is
indicated by the development and presence of spermatophores (Filina 2011). However, for
regulatory purposes, ADF&G simply defines “mature” as meaning “male red king crab that are
4.7 inches (120 mm) or more in carapace length” (ADF&G 2023). For male RKC, we will
follow that convention to facilitate comparisons between results of the current survey and both
ADF&G and NMFS documents describing similar aspects of the stock. By extension, we will
refer to all male RKC that are < 120 mm (< 4.7 in) CL as “immature-size”, regardless of their
functional abilities to reproduce. Finally, males ≥ 135 mm (5.3 in) CL will be referred to as
12
“legal-size” males, as this currently represents the minimum size for retention in the directed
fishery.
All RKC were visually inspected for signs of disease and, if noted, the apparent nature of the
disease was recorded. Specifically, crab were scanned for the following pathologies: 1) bacterial
shell disease (Meyers and Burton 2009); 2) rhizocephalan barnacles (esp. Briarosaccus spp.;
Sheilds 2012, Sloan and Hardy 2017); 3) cottage cheese disease (i.e., microsporidian infection;
Stentiford et al. 2014); 4) leatherback (i.e., incomplete calcification of the exoskeleton; Morado
et al. 2014), and; 5) snailfish (Liparidae) eggs (Gardner et al. 2016).
Bycatch Sampling
At each station, all crabs were sorted by species. Hybrid crabs representing a cross between
Tanner and snow crab were identified by a combination of characteristics including curve of the
epistome margin, eye color, carapace shape, and space between or shape of the rostrum horns
(Karinen and Hoopes 1971, Urban et al. 2002). Tanner crabs, snow crabs, and Tanner-snow
hybrids were enumerated by sex. Fish were enumerated by species and non-crab invertebrates
were identified to lowest known taxa.
Red King Crab Tagging
Wildlife Computers (Redmond, Washington, USA) Pop-up Archival Transmitting (PAT) tags
were deployed on 100 mature-size male RKC that had recently molted and were in a new hard-
shell condition. Two models of tag were used: 20 miniPATs; and 80 mrPAT “mark-report” tags.
The miniPAT measures 118 mm (4.64 in) in length by 38 mm (1.50 in) in maximum diameter,
with a plastic-coated braided-cable antenna protruding from the distal end. The miniPAT is
capable of recording temperature, depth, and ambient light levels for periods of up to 2 years at
recording intervals of one minute; or recording at longer or shorter logging intervals depending
upon intended deployment duration. The mrPAT is somewhat smaller, measuring 118 mm (4.64
in) by 28 mm (1.10 in) and records daily minimum and maximum temperature and tag-tilt. Only
crab with no, or very minimal injuries (e.g., spine breaks), were selected for tagging. Crabs that
13
met the tagging criteria were placed in a tank with flowing seawater as soon as possible upon
capture and there awaited tagging. The minimal tagging criteria required five new hard-shell
mature males to be caught within a 5-pot string of gear. To distribute tags across the entire
survey grid, one tag was deployed every other 5-pot string when the minimal criteria were met.
In addition, more tags were placed at sites that met the hotspot criteria. If a 5-pot string captured
> 100 new hard-shell mature males, five crab were tagged from that string. A total of four
“hotspots” of crab catch were identified. Following completion of all of the standard survey
stations, undeployed tags remained; thus, to increase tagging density at hotspot sites, pots were
reset in hotspot areas, tagging a total of 13 to 15 crab within each hotspot. Tags were attached to
crabs by means of a polyolefin tubing harness that wrapped around the crabs’ carapace, going
around the second walking legs (Fig. 8). The positively buoyant tag floats approximately 7 cm
(2.75 in) above the crab.
Graphical and Statistical Analyses
For NMFS summer trawl survey data, total crab abundance is estimated annually from the raw
survey data using area-swept methods (Zacher et al. 2024) and subsequent quantitative stock
assessment modeling (Palof and Siddeek 2022). Area-swept methods cannot be applied to pot
survey data, and drawing similar inferences based on pot data would require, among other
factors, a refined understanding of the attractive radius and distance-dependent fishing power of
the traps (sensu Aedo and Arancibia 2003) under a variety of underlying environmental
conditions. Quantitative abundance estimation methods based on pot/trap data do exist for a
limited number of crab stocks: management of Florida stone crab (Menippe spp.) has relied on
surplus production modeling based on commercial catch data from the trap fishery (Muller et al.
2006) and assessment models based on pot surveys have been employed for southeast Alaska
RKC (see Quinn II et al. 2006). However, no such models exist for BBRKC and developing
abundance estimation techniques for this stock based on pot survey data would require surveys
that more-fully encompass the geographic range of the stock as well as multiple years of survey
data. Herein, we will make no attempt to estimate the absolute or relative abundance of any
demographic of RKC sampled and will instead simply report results as total numbers of crabs
captured within each sex, size, and maturity category of interest and the ratios of total catch

14
among those demographics. Raw temperature data were interpolated for the survey area via
ordinary kriging using R statistical software (R v.4.2.2; R Core Team 2023). Where means
(averages) are reported, errors will represent on standard deviation about the mean, unless
otherwise noted.
Public Data Repository
Data generated during the course of this survey have been made publicly available and may be
accessed from NMFS’ Alaska Fisheries Science Center Shellfish Assessment Program’s GitHub
site.
RESULTS
Survey Completion
A total of 637 standard survey stations were fished between 18 March and 4 April 2023; 59
planned stations were not fished due to logistical considerations (e.g., location too shallow) and
time constraints (Fig. 3). One additional station was dropped from the final dataset because the
pot had been left unbaited. Among fished stations, 299 were fished by the FV Summer Bay and
338 by the FV Silver Spray (Fig. 3). Realized station coordinates resulted in a total of 99 stations
within the RKCSA (i.e., 15.7% of stations fished), 24 stations (3.7%) within the RKCSS, and
271 stations (42.5%) within the NBBTCA; all stations were within BLZ1. Station depths ranged
from 14.0 to 50.6 fm (25.6 – 92.5 m). Ideally, soak time at each station would have been
approximately 30 hours; however, logistics associated with weather, processing of catch, and
running time between stations imparted variance around the ideal. Realized soak times ranged
between 27.5 and 79.1 hours with the majority of soak times (92%; n = 586) falling within a
positively-skewed distribution (Fig. 9) that spanned 27.5 – 48.8 hours. Falling to the right of (i.e.,
longer than) the primary distribution of soak times were two additional groupings: one composed
of 20 stations with soak times ranging from 52.8 to 53.1 hours; and a second group composed of
29 stations with soak times of 69.9 – 79.1 hours (Fig. 9).
15
In addition to the standard survey stations, 47 experimental potlifts were conducted, as follows:
1) 30 potlifts were conducted from 3 to 6 April on survey Transects C, E, and I to collect
additional crabs for tagging; 2) 5 potlifts were conducted between 21 March and 3 April to
compare catch rates using varying volumes (i.e., two and four bait bags) and types of bait (i.e.,
herring and cod) and to observe catches using underwater cameras, and; 3) 12 potlifts conducted
on 5 April for tagging. The results and graphics that follow will not include the additional
stations; they will be limited to the 637 potlifts conducted at standard survey stations.
Oceanographic Conditions
Temperature loggers were deployed at 38.6% (n = 246) of standard stations fished, distributed
fairly evenly across the survey area (Fig. 10, lower). Mean water temperatures (i.e., averaged
over the course of each pot soak when the pots were determined to be on-bottom) at individual
stations ranged from -1.22 to 4.38° C (29.84 – 39.88° F) and averaged 2.02 ± 1.08° C (35.06 ±
1.94° F). The warmest temperatures were observed in the southwest of the survey area, closest to
deepwater habitat north of Unimak Pass, and coldest temperatures were encountered in the
northwest, in relatively shallow habitat southwest of Cape Newenham (Fig. 10, lower). Overall,
bottom temperatures in the surveyed region were cooler than observed during the NMFS EBS
summer trawl surveys that were conducted before and after the pot survey (i.e., during the
summers of 2022 and 2023; Fig. 10, upper). Although sea ice was not encountered at any of the
pot survey stations, relatively dense ice coverage was reported just north of the survey grid
during the survey’s first week and the ice retreated to waters offshore and west of Cape
Newnham, and along the Alaska Peninsula in the Kvichak Bay region, by the time the survey
was completed (Fig. 11). During sampling, the footprint of the associated cold pool extended
into the survey grid in its northwest corner (Fig. 10, lower).
Red King Crab Catch Composition
A total of 10,191 RKC were captured (Table 1) at 450 (70.5%) of the standard survey stations:
76.8% (n = 7,824) of these crabs were male and 33.2% (n = 2,367) were female. Considering
only mature-sized individuals, the observed sex ratio was 72.1% male. This departs substantially
16
from recent EBS trawl survey results, in which catch throughout the Bristol Bay District was
roughly 52% male in 2022 and 37% male in 2023 (Zacher et al. 2024). Male RKC that were
captured at standard survey stations ranged from 17.6 to 190.2 mm (0.69 – 7.49 in) CL; the size
of one individual was not obtained. Among the measured individuals, 36.1% (n = 2,824) were
immature-size (< 120 mm (4.7 in) CL); 63.9% (n = 4,999) were mature-size (≥ 120 mm (4.7 in)
CL); 44.7% (n = 3,497) were legal-size (≥ 135 mm (5.3 in) CL). No molting males were
observed. Immature males were predominantly (98.8%) new hardshell with a small proportion
(1.1%) of old shell and three individuals with very old shell (Fig. 12). The occurrence of old
shell condition increased with size for males larger than ~125 mm (4.92 in) CL, such that in
mature-size males the proportion of new hard decreased to 57.6%, old shell increased to 38.1%,
and 4.2% of individuals were very old shell. Among legal-size males, the proportion of new hard
and old shell were similar (48.5% vs. 45.8%, respectively) and old shell was 5.7%; these were
similar to the relative proportions reported by Zacher et al. (2024) for the 2023 trawl survey.
Across all sizes, a much smoother progression in the advancement of shell conditions with size
can be seen in the CPS1 data than has been visible in recent trawl survey data (Fig. 12).
Female RKC ranged from 57.0 to 174.6 mm (2.24 – 6.87 in) CL: 81.7% (n = 1,934) of these
individuals were determined to be morphometrically mature. Immature females ranged in size
from 57.0 to 99.9 mm (2.24 – 3.93 in) CL and mature females from 82.1 to 174.6 mm (3.23 –
6.87 in) mm CL (Fig. 13). Only five molting individuals were captured, all of which were
immature. The remainder of immature females (98.9%) were new hardshell. Among
morphometrically mature females, 47.6% were new hard-shell and 52.2% were old shell.
Overall, a substantially larger proportion of females were old shell than observed during recent
summer surveys (Fig. 14), especially at sizes greater than ~ 100 mm (3.84 in) CL. No barren
mature females were encountered: all morphometrically-mature females bore egg clutches. No
clutches contained dead eggs, hatching embryos, or empty egg cases. Clutches were composed of
44.3% and 55.7% uneyed and eyed eggs, respectively. The proportion of females carrying eyed
versus uneyed eggs increased progressively with size, with eyed eggs rarely seen in females < 93
mm (3.66 in) CL while representing the large majority of individuals >115 mm (4.53 in) CL
(Fig. 15). Similarly, clutch fullness followed a clear progression of increasing volume with crab
length (Fig. 16), in which half-clutches were represented by a small proportion of individuals
17
(3.4%) primarily ranging from 84 to 104 mm (3.31 – 4.09 in) mm CL; females carrying three-
quarter clutches produced a strongly regular distribution across individuals of 82 – 119 mm (3.23
– 4.69 in) CL; and full clutches were carried by the majority of individuals ≥ 110 mm (4.33 in)
CL. Overall, 43.5 % of individuals carried three-quarter egg clutches and 53.0% carried full
clutches.
Red King Crab Spatial Distribution
Overall, RKC were distributed throughout the survey area except in its southwestern corner
(Fig. 17, lower): RKC were largely absent south and west of a line running from roughly
56° 50’ N lat. × 164° 15’ W long. southeastward to 52° 50’ N lat. × 162° 27’ W long. Zero-catch
stations were also common on the southern ends of the transects from the Black Hills eastward,
with the exception of the easternmost transect (Transect K; Fig. 17, lower) that terminated
slightly farther offshore than on Transects E-J. The observation of relatively low overall
abundance in the southwest was similar to that which was observed during the 2022 and 2023
NMFS EBS summer trawl surveys (17, upper).
The distributions of legal-size (Fig. 18, lower) and mature-size (Fig. 19, lower) males were
similar to one another, as well as to the distribution of all RKC. Immature-size males displayed a
somewhat patchier distribution than the larger males, especially within the survey’s western
transects (Fig. 20, lower). However, they still possessed an overall footprint that was similar to
that of the larger males. For females, there was a greater disparity between the distribution of
mature and immature individuals. Mature females (Fig. 21, lower) displayed a broad distribution
that was similar to that of males. Immature females were largely absent from the four western-
most transects as well as the survey’s northeastern extent (Fig. 22, lower). Immature females
were largely concentrated to the northeast of the RKCSA, with one “hotspot” located inside the
NBBTCA and a second just north of the RKCSA boundary.
Across all demographics, the majority of crab were caught within the NBBTCA (Table 1): 66%
of all RKC were captured in the NBBTCA and percentages ranged from 61.7% (legal-sized
males) to 75.8% (mature females) among individual crab demographics. For males, just under
18
20% of each demographic was encountered in the RKCSA, as well as in the BLZ1 outside of the
RKCSA and NBBTCA. For females, the proportion of crab captured inside the RKCSA was
quite different for mature (17.4%) versus immature (7.6%) crabs. A considerably higher
proportion of immature females (21.5%) was found outside of the RKCSA and to the west of the
NBBTCA boundary than mature females (6.8%). This difference in proportional catch appears to
have been due more to an absence of immature females in the RKCSA than due to an abundance
of immature females in BLZ1 outside of the RKCSA.
For no plotted demographic did the overall spring distribution derived from CPS1 data appear
markedly different than the distribution found during the 2023 NMFS summer trawl survey.
However, for immature crabs of both sexes, the CPS1 distributions appear more similar to the
2023 trawl survey plots than to 2022. For crabs of mature and legal sizes, pot survey
distributions appear somewhat less patchy than suggested by the trawl survey data.
Other Crab Species
In addition to RKC, four distinct species of crabs were captured: Tanner crab, snow crab,
horsehair crab (Erimacrus isenbeckii), and Pacific lyre crab (Hyas lyratus). Additionally, a small
number (n = 4) of Tanner-snow crab hybrids were captured. Tanner crabs were the most
abundant (Table 2), represented by 570 individuals captured at a total of 227 stations; most
(98.1%) of these crabs were male. All snow crab and Tanner-snow hybrids were male and
occurred at considerably lower abundance and at fewer stations than Tanner crabs (Table 2).
Tanner crabs were distributed throughout the survey region, with the greatest concentration in
the western half of the grid in a triangle comprising the deepest water and extending
northwestward, roughly parallel to the 100-m isobath (Fig. 23). Snow crabs (Fig. 24) were found
only in the western half of the survey grid, in a distribution that largely overlapped that of Tanner
crab, but with a smaller total footprint.

19
Fish and Macroinvertebrates
The most commonly encountered fish species (Table 2) were Pacific cod (n = 1,728 individuals
captured at 510 stations) and yellowfin sole (n = 2,393 individuals from 420 stations). Great
sculpin (Myoxocephalus polyacanthocephalus; n = 78) were captured at 69 stations.
Additionally, small numbers of walleye pollock (Gadus chalcogrammus), Pacific halibut
(Hippoglossus stenolepis), northern rock sole (Lepidopsetta polyxystra), starry flounder
(Platichthys stellatus), Alaska plaice (Pleuronectes quadrituberculatus), Alaska skate (Bathraja
parmifera), unidentified skates (Rajidae), and sunflowers seastars (Pycnopodia helianthoides)
were encountered (Table 2).
Pacific cod were broadly distributed throughout the survey grid (Fig. 25) but displayed
somewhat lower abundance along an east-west axis through the central RKCSA and westward
into BLZ1 than elsewhere. Pacific cod were absent from stations on the northern end of
Transects A-D, where the coldest temperatures were recorded. Yellowfin sole were also broadly
distributed and found at highest abundance in nearshore waters along the Alaska Peninsula and
along, and just outside of, the 50-m isobath in northern Bristol Bay (Fig. 26).
Red King Crab Tagging
Tags deployed during CPS1 had a 91% success rate in providing reliable locations with minimal
error ellipses (Fig. 27). New hard-shell mature male RKC had an average movement rate of 0.83
± 0.50 km/day (0.45 ± 0.27 nmi/day), with a range of 0.08 to 2.53 km/day (0.04 to 1.37 mi/day),
over 57–75 days at liberty. There was a high degree of variability in direction of movement (Fig.
27), but with a prevailing trend of movement to the north and northeast (Fig. 28). Tags released
from crabs and reported their locations, providing information that is coincident with the NMFS
eastern Bering Sea trawl survey in Bristol Bay. Survey results and tagging show the same
general area occupied in Bristol Bay (Fig. 29). Additional analyses of finer-scale patterns in
distribution are underway.
20
DISCUSSION
The current study was largely successful in demonstrating the feasibility of conducting pot
sampling for BBRKC in early spring, thereby generating data on demographic composition,
relative abundance, and stock distribution at a time of year when data for the population have
generally been unavailable. Additionally, the sampling was explicitly designed and executed to
target and sample RKC, without the need to simultaneously survey a wide range of groundfish
species. As a result, considerably more crabs were encountered and sampled during CPS1 than
during the subsequent EBS trawl survey, including nearly three times as many mature females
within the CPS1 grid than were captured during the trawl survey within the Bristol Bay District.
As such, many important aspects of RKC biology may be better-described by the current data
than most years’ trawl surveys have been able to generate. In general, changes in shell condition,
maturity, and egg production according to crab size occurred in considerably clearer progression
within the CPS1 length-frequency plots. Such data can allow for more thorough analyses and
novel insight into aspects of RKC ecology that are difficult or impossible to obtain from summer
trawl survey data. For example, the length or age at which 50% of individuals within a
population become mature (i.e., L50) and the range of sizes over which individuals in a
population reach maturity are important life history parameters used to manage populations and
evaluate their responses to both fishing pressure (Sharp and Hendry 2009) and environmental
change (e.g., McLeay et al. 2019). Ideally, the data describing the maturation process will follow
a smooth sigmoidal (S-shaped) progression over the maturing sizes, thereby allowing confident
estimation of the maturation curve’s inflection point (= L50); such a form can be seen in the
CPS1 data for female RKC (Fig. 12). Ultimately, multiple years of trawl survey data might be
combined to generate a reasonable fit to a sigmoidal maturation curve. However, it can be
unsatisfying and imprecise to pool data over multiple years and derive a longer-term mean when
populations are subjected to environmental change, such that maturation dynamics may change
within the period described by the data pooling.
In most years, the summer trawl survey occurs when molting and mating have been largely
completed, providing an understanding of the results of the most-recent breeding season but less
insight into active spawning and reproductive dynamics within the breeding season. Inspection
21
of the CPS1 data suggest that it fell largely in the midst of spawning, when primiparous females
had molted and mated and multiparous females were largely preparing to spawn. The length-
frequency distributions depicting shell (Fig. 14) and egg (Fig. 15) condition contain a clear mode
of small crab that had molted and were in new hard-shell condition bearing clutches of recently
extruded uneyed eggs, while most of the larger females had old shells, eyed eggs, and none had
empty egg cases, indicating that they would likely molt soon. The ability to put survey platforms
on the water early in the year can allow for the collection of data that may improve our
understanding of reproductive dynamics, as well as providing data on changes in distribution on
seasonal scales. At the same time, it is important to recognize that the structure of catches may
vary seasonally and across gear types as a function of changes in selectivity, especially as
influenced by the behavior of the crabs. This can be particularly true for baited gear, as changes
in feeding motivation and individuals’ willingness to interact with sampling gear can be
influenced by a variety of factors including age, sex, physiological condition, and breeding
status. In the current survey, of particular note was a substantially different sex ratio in pot
catches relative to that which is commonly observed during summer trawl surveys. The pot
survey results were biased in favor of males to a much greater degree than trawl survey data and
produced a clear lack of molting and recently molted females despite evidence (i.e., the
progression of shell and egg condition classes) that egg hatch and molting was likely ongoing.
For commercially exploited crustaceans, apparent changes in catchability according to size, sex,
and season have been observed elsewhere when sampled using traps. For example, using both
trammel nets (i.e., a form of benthic gillnet) and traps to survey Mediterranean spiny lobsters
(Palinurus elephas), Goñi et al. (2003) observed clear differences in male and female catch rates
based on season. Female catch was quite similar between sampling gears and remained relatively
constant throughout the year. However, male size structure was represented differently between
traps and nets and the relative proportion of males captured followed a seasonal progression in
which males were highly under-represented during the molting period and displayed greatest
catchability when spawning. In Mediterranean spiny lobster, spawning takes place while both the
males and females are hard-shelled, during the intermolt period a few weeks after female molting
(Yeap et al. 2022). Trap-shyness during molting in this species appears to be sex-specific and
displayed primarily by males. For females, the authors (Goñi et al. 2003) hypothesized that
22
shelter-seeking behavior and their tendency to be more gregarious might enhance their
catchability year-round. The result of the divergent sex-specific behaviors will thus change the
inferred sex ratio of the population as derived from pot sampling, especially among reproductive
size classes, even though little change is likely to be occurring in the underlying population. In
contrast to spiny lobsters, Brunson et al. (2023) found that pots consistently produced a higher
male sex ratio than trawl sampling for blue crab (Callinectes sapidus) in Chesapeake Bay
estuaries. The male bias was further accentuated in summer and winter relative to spring and
autumn. While the authors were unable to explain the apparently greater affinity of males for
pots overall, they hypothesized that the seasonal variance in sex ratios was related to changes in
female catchability due to breeding behavior: in particular, increased catch rates of females when
they are preparing to molt and breed. Sublegal-size females in a pre-molt status (i.e., “peeler”
crabs) are attracted to mature males. Peeler crabs represent an important target demographic for
the commercial fishery because they are held after capture and allowed to molt into marketable
soft-shells (Huang et al. 2015) and the peeler-crab fishery often “baits” its pots with large mature
males in order to increase female peeler catches (Bishop et al. 1983). In cases in which mature
males enter the traps of their own accord during the spawning season, the result may be a marked
increase in female catchability within those pots and the false perception that the underlying sex
ratio has changed.
In the current survey, the general lack of molting individuals in concert with a much lower
proportion of females than is estimated from trawl-survey data is consistent with female pot
shyness. However, closer inspection suggests the results may reflect pot shyness in both sexes
combined with earlier molting in males. The only molting individuals that were captured were
females, despite having caught more than three times as many males. There is no evidence in the
CPS1 data that molting males were inclined to enter the pots. Rather, if females molt later than
males, which is consistent with RKC mate-guarding behavior (Powell et al. 2002) in which
males must be hard-shell either in advance of the females or well afterwards in order to ensure
their ability to clasp and guard their molting mate, then it is likely that the population was
composed of a much higher proportion of molting females than molting males and the females
were therefore underrepresented in the catch. Regardless of the ultimate mechanism (i.e.,
whether pot shyness is sex-specific or the catches were a result of differential molt timing), a
23
survey that is biased against females could have consequences in terms of the management
actions that might be suggested using the data derived from it. Although the current survey was
not intended to estimate total abundance of any demographic of the population, one of its
objectives was to investigate the spatial distribution of each demographic outside of the summer
season and produce information that might be adapted for use by trawl fleets to avoid RKC
bycatch during winter-spring fisheries. At its June 2023 meeting, the NPFMC moved to “explore
further action that could be implemented through framework agreements for the pot CV sector
and trawl sectors … to reduce BBRKC mortality … responsive to seasonal spatial distribution of
BBRKC and focus avoidance on more discrete areas of relatively higher female BBRKC
abundance” (NPFMC 2023). In order for such guidance to be effective, the data describing
female distribution must be accurate to the greatest degree possible. If pots under-sample females
in late winter and early spring, potentially due to pot shyness associated with molting and
mating, complications and inaccuracies could arise, with the nature of those inaccuracies being
dependent upon the effect of pot-shyness on inferred distribution patterns. For example, if pot-
shy, molting females are distributed evenly throughout the underlying population, then the effect
of undersampling those individuals may simply be a down-scaling of apparent total abundance,
but the data would still provide an accurate representation of relative distribution and the location
of “hotspots” that bycatch fisheries might avoid. However, if the distribution of pot-shy females
is patchy, then the relative distribution inferred from pot sampling may be different than the true
underlying distribution. In particular, failure to locate and characterize aggregations of molting
females that are likely to be the more vulnerable to handling mortality than hard-shell individuals
(Stevens 1990) could create the possibility of guiding bycatch fisheries into the areas where
those crabs exist, instead of avoiding them, simply due to a lack of informative data regarding
their locations. At present, there is no way to infer from the existing data whether female catches
in the current survey were proportional to their underlying distribution or departed from it, or, if
catches were not fully representative of underlying spatial distribution, to what degree they may
have failed to represent specific size class(es), shell, and clutch conditions. Future sampling
should seek to address this issue by including additional, paired sampling using techniques free
from this specific bias (sensu Hanamseth et al. 2022), thereby allowing for a comparison of the
structure and distribution of catches between or among sampling techniques.
24
In addition to addressing apparent pot shyness, future sampling should seek to standardize pot
gear among vessels, to the greatest degree practicable, in order to avoid any potential spatial
biases within the survey region. For example, the current survey employed pots that had been
manufactured using slightly different webbing sizes on the pots’ main body and funnels. In all
cases, the webbing sizes were considerably smaller than employed during commercial fishing
operations and so their ability to retain large males and mature females is unlikely to have
differed. However, if one were interested in indexing small, pre-recruit individuals then it is
possible that even small differences in webbing sizes could result in slightly different selectivity,
which could translate into perceived spatial variance in the apparent abundance of the smallest
crabs even if no such variance existed. This could be accentuated if pots of different
configuration are used within discrete spatial “blocks” within a survey, such that the selectivity
differences have discrete spatial footprints. For example, in the current survey, a north-south
difference in the perceived abundance or distribution of small crabs could arise due to one vessel
having fished the northern half of the grid with pots that were of slightly different configuration
than the pots used in the southern half of the grid. Ideally, all pots used for future work should be
standardized to ensure equivalent selectivity and fishing power across all demographics of
potential interest, including standardization of pot size, funnel design, webbing sizes; and bait
type, source, quality, and quantity. In cases in which complete standardization is impractical,
differences in selectivity among pot configurations could be examined (e.g., Zhou and Kruse
1999, Herrmann 2021) and accounted for by conducting paired sampling at individual stations to
quantify any differences and applying selectivity coefficients to survey data post hoc to
standardize the resulting catches (sensu Gibson-Reinemer et al. 2017). However, this can be a
large undertaking necessitating dedicated studies and often cannot be directly introduced into the
design of surveys, themselves. Alternatively, if all pots cannot be standardized to a single strict
configuration, then one might elect to evenly distribute pot configurations among all vessels and
deploy them in either a randomized or orthogonal design that eliminates the potential for
systematic spatial bias in catches.

25
ACKNOWLEDGMENTS
This project was funded primarily by the Alaska Department of Fish and Game and the National
Marine Fisheries Service, with additional support from the Bering Sea Fisheries Research
Foundation. We thank Captain Bill Prout of the FV Silver Spray and Captain Mike Wilson of the
FV Summer Bay and all their crew whose hard work and expertise made this a safe and
successful survey. Katie Palof (ADF&G), Chris Siddon (ADF&G), Gary Stauffer (BSFRF),
Gordon Kruse (BSFRF), and Madison Heller-Shipley (BSFRF) provided guidance during the
project’s design and planning phases. Allie Conrad (NMFS-AFSC), Connor Cleary (NMFS-
AFSC), and Rachel Alinsunurin (ADF&G) provided assistance in gear preparation. Danielle
Lampe (ADF&G) and Jon Richar (NMFS-AFSC) aided in data processing. In addition to the co-
authors who served aboard the survey vessels (E. Ryznar and L. Zacher, field party chief on
Silver Spray), we acknowledge the dedication of the scientific personnel who made up the 2023
CPS1 “crab crew”: Charlie Heller-Shipley (Natural Resources Consultants / BSFRF), Cory
Lescher (Alaska Bering Sea Crabbers / BSFRF), Vicki Vanek (ADF&G), and Jared Weems
(ADF&G, field party chief on Summer Bay).
26
27
CITATIONS
Adams, A. E., and Paul, A. J. 1983. Male parent size, sperm storage and egg production in the
crab Chionoecetes bairdi (Decapoda, Majidae). International Journal of Invertebrate
Reproduction 6(3):181-187. DOI: 10.1080/01651269.1983.10510040
Aedo, G., and Arancibia, H. 2003. Estimating attraction areas and effective fishing areas for
Chilean lemon crab (Cancer porteri) using traps. Fisheries Research 60(2-3):267-272. DOI:
10.1016/S0165-7836(02)00177-7
Alaska Department of Fish and Game (ADF&G). 2023. 2023 – 2024 Statewide King and Tanner
Crab Commercial Fishing Regulations. Alaska Department of Fish and Game, Juneau, USA.
207 p. Available:
https://www.adfg.alaska.gov/static/regulations/fishregulations/pdfs/commercial/cf_king_
tanner_crab_2023_2024.pdf
Baker, K. D., Mullowney, D. R., and Saint-Marie, B. 2022. Large males matter: low sperm
reserves in female snow crab (Chionoecetes opilio) off Newfoundland, Canada. Fisheries
Research 253(2022):106385. DOI: 10.1016/j.fishres.2022.106385
Bishop, J. M., Olmi, E. J. III., Whitaker, J. D., and Yianopoulos, G. M. 1983. Capture of blue
crab peelers in South Carolina: an analysis of techniques. Transactions of the American
Fisheries Society 112:(1):60-70. DOI: 10.1577/1548-8659(1983)112<60:COBCPI>2.0.CO;2
Block, A. B., Dewar, H., Williams, T., Prince, E. D., Farwell, C., and Fudge, D. 1998. Archival
tagging of Atlantic bluefin tuna (Thunnus thynnus thynnus). Marine Technology Society
Journal 32(1):37-46. DOI: 10.1007/978-94-017-1402-0_3
Bryan, D. R., McDermott, S. F., Nielsen, J. K., Fraser, D., and Rand, K. M. 2021. Seasonal
migratory patterns of Pacific cod (Gadus macrocephalus) in the Aleutian Islands. 2021.
Animal Biotelemetry 9:24 (2021). DOI: 10.1186/s40317-021-00250-2
Brunson, J. F., Sitta, K. A., Kendrick, M. R., and Kingsley-Smith, P. R. 2023. Evidence in male
bias in Atlantic blue crab pot-based sampling. bioR
x
iv (preprint). DOI:
10.1101/2023.05.09.538440v1
28
Cates, K., Marrinan, S., and Smith, M. 2022. Considering a closure to the Red King Crab
Savings Area for all gear types. Discussion Paper C1 RKC Savings Area December 2022.
North Pacific Fishery Management Council, Anchorage, USA. Available:
https://meetings.npfmc.org/CommentReview/DownloadFile?p=80d47407-c90a-44ca-997a-
fcc8c0b7d5cc.pdf&fileName=C1%20Red%20King%20Crab%20Savings%20Area%20Anal
ysis.pdf
Cunninham, S., and Cates, K. 2022. Bristol Bay red king crab information. North Pacific Fishery
Management Council Discussion Paper D1 BBRKC Info Paper April 2022. North Pacific
Fishery Management Council, Anchorage, USA. Available:
https://meetings.npfmc.org/CommentReview/DownloadFile?p=7608c5c6-d20a-4b3e-a23a-
7fb0754d3f71.pdf&fileName=D1%20BBRKC%20Information%20Paper.pdf
Donaldson, W. E., and Beyersdorfer, S. 2003. Biological field techniques for lithodid crabs.
Alaska Sea Grant College Program AK-SG-05-03, University of Alaska, Anchorage, USA.
76 p.
Donaldson, W. E., and Beyersdorfer, S. 2014. Anatomy of king crabs, p. 73-80. In Stevens, B. G.
King (eds.), King Crabs of the World: Biology and Fishery Management. CRC Press, New
York, USA.
Filina, E. A. 2011. Spermatogenesis and physiological maturity of male red king crab
(Paralithodes camtschaticus Tilesius, 1815) and snow crab (Chionoecetes opilio Fabricius,
1788) in the Barents Sea. Marine Biology Research 7(3):289-296. DOI:
10.1080/17451000.2010.497188
Ganji, P. C. N. 2011. Reproductive regulators in decapod crustacean: an overview. Journal of
Experimental Marine Biology and Ecology 214:3-16. DOI: 10.1242/jeb.047183
Gardner, J. R., Orr, J. W., Stevenson, D. E., Spies, I., and Somerton, D. A. 2016. Reproductive
parasitism between distant phyla: molecular identification of snailfish (Liparidae) egg
masses in the gill cavities of king crabs (Lithodidae). Copeia 104(3):645-657. DOI:
10.1643/C1-15-374

29
Gibson-Reinemer, D. K., Ickes, B. S., and Chick, J. H. 2017. Development and assessment of a
new method for combining catch per unit effort data from different fish sampling gears:
multigear mean standardization (MGMS). Canadian Journal of Fisheries and Aquatic
Sciences 74(1):8-14. DOI: 10.1139/cjfas-2016-0003
Goñi, R., Quetglas, A., and Reñones, O. 2003. Differential catchability of male and female
European spiny lobster Palinurus elephas (Fabricius, 1787) in traps and trammel nets.
Fisheries Research 65:295–307. DOI: 10.1016/j.fishres.2003.09.021
Hanamseth, R., Johnson, D. D., Schilling, H. T., Suthers, I. M., and Taylor, M. D. 2022.
Evaluation of a novel research trap for surveys of blue swimmer crab populations. Marine
and Freshwater Research 73(6):812-822. DOI: 101071/MF21005
Herrmann, B., Grimaldo, E., Brčić, J., and Cerbule, K. 2021. Modelling the effect of mesh size
and opening angle on size selection and capture pattern in a snow crab (Chionoecetes opilio)
pot fishery. Ocean and Coastal Management 201(2021):105495. DOI:
10.1016/j.ocecoaman.2020.105495
Hernández-Sandoval, P., Díaz-Herrera, F., Díaz-Gaxiola, J. M., Martínez-Valenzuela, C., and
Garía-Guerrero, M. G. 2018. Effect of temperature on growth, survival, thermal behavior,
and critical thermal maximum in the juveniles of Macrobranchium occidentale (Holthius,
1950) (Decapoda: Caridea: Palaemonidae) from Mexico. Journal of Crustacean Biology
3(4):483-488. DOI: 101093/jcbiol/ruy024
Huang, P., Woodward, R. T., Wilberg, M. J., and Tomberlin, D. 2015. Management evaluation
for the Chesapeake Bay blue crab fishery: an integrated bioeconomic approach. North
American Journal of Fisheries Management 35(2):216-228. DOI:
10.101080/02755947.2014.986342
Jørgensen, C., Ernande, B., Fiksen, Ø, and Diechmann, U. 2006. The logic of skipped spawning
in fish. Canadian Journal of Fisheries and Aquatic Sciences 63(1):186-199. DOI:
10.1139/f05-210
30
Karinen, J., and Hoopes, D. 1971. Occurrence of Tanner crabs (Chionoecetes bairdi) in the
eastern Bering Sea with characteristics intermediate between C. bairdi and C. opilio.
Proceedings of the National Shellfish Association 61:8-9.
Keating, K. A. 1995. Mitigating elevation-induced errors in satellite telemetry locations. Journal
of Wildlife Management 59:801-808. DOI: 10.2307/3801960
Kotwicki, S., Buckley, T. W., Honkalehto, T., and Walters, G. 2005. Variation in the distribution
of walleye pollock (Theragra chalcogramma) with temperature and implications for
seasonal migration. Fishery Bulletin, U.S. 103(4):574-587.
Loher, T. 2022. Dispersal and seasonal movements of Pacific halibut (Hippoglossus stenolepis)
in the eastern Bering Sea and Aleutian Islands, as inferred from satellite-transmitting
archival tags. Animal Biotelemetry 10:18. DOI: 10.1186/s40317-022-00288-w
McLeay, L. J., Doubell, M. J., and Linnane, A. J. 2019. Spatial and temporal variations in female
size at maturity of a southern rock lobster (Jasus edwardsii) population: a likely response to
climate change. PLosOne 14(11):e0225144. DOI: 10.1371/journal.pone.0225144
Meyers, T., and Burton, T. 2009. Diseases of Wild and Cultured Shellfish in Alaska. Alaska
Department of Fish and Game, Fish Pathology Laboratories, Anchorage, USA. 130 p.
Morado, J. F., Shavey, C. A., Ryazanova, T., and White, V. C. 2014. Diseases of king crabs and
other anomalies, p. 139-210. In Stevens, B. G. (ed.), King Crabs of the World: Biology and
Fishery Management. CRC Press, New York, USA.
Muller, R. G., Bert, T. M., and Gerhart, S. D. 2006. The 2006 Stock Assessment Update for the
Stone Crab, Menippe spp., Fishery in Florida. Florida Fish and Wildlife Conservation
Commission, Florida Marine Research Institute, St. Petersburg, USA. Available:
https://sjrda.stuchalk.domains.unf.edu/files/content/sjrda_464.pdf
Nielsen, J. K., Mueter, F. J., Adkinson, M. D., Loher, T., McDermott, S. F., and Seitz, A. C.
2019. Effect of study area bathymetric heterogeneity on parameterization and performance
of a depth-based geolocation model for demersal fishes. Ecological Modeling 24:18-34.
DOI: 10.1016/j.ecolmodel.2019.03.023
31
Nichol, D. G., and Somerton, D. A. 2015. Seasonal migrations of morphometrically mature male
snow crabs (Chionoecetes opilio) in the eastern Bering Sea in relation to mating dynamics.
Fishery Bulletin, U.S. 113(3):313-326. DOI: 10.7755/FB.113.3.7
North Pacific Fishery Management Council (NPFMC). 2023. C- 4 BBRKC closure area. Council
Motion 1 BBRKC Closure Area, 11 June 2023. North Pacific Fishery Management Council,
Anchorage, USA. Available:
ttps://meetings.npfmc.org/CommentReview/DownloadFile?p=c82d4c84-8e06-4dde-8a14-
c9777cfbde86.pdf&fileName=C4%20Motion%201%20BBRKC%20Closure%20Area.pdf
Olsen, A. P. 2016. Spatial Variability in Size at Maturity and Reproductive Timing of Golden
King Crab (Lithodes aequispinus) in Southeast Alaska. Master’s Thesis. University of
Alaska, Fairbank, USA. 55 p.
Palof, K. J., and Siddeek, M. S. M. 2022. Bristol Bay Red King Crab Stock Assessment in Fall
2022. North Pacific Fishery Management Council, Anchorage, USA. 187 pp. Available:
https://meetings.npfmc.org/CommentReview/DownloadFile?p=b98b90b2-88ab-43c2-9487-
c12cdb4e0a25.pdf&fileName=BBRKC%20SAFE%202022%20Final.pdf
Pederson, M. W., Righton, D., Thygesen, U. H., Andersen, K. H., and Madsen, H. 2018.
Geolocation of North Sea cod (Gadus morhua) using hidden Markov models and
behavioural switching. Canadian Journal of Fisheries and Aquatic Sciences 65(11):2367-
2377. DOI: 10.1139/F08-144
Powell, Pengilly, D., and Blau, S. F.. 2002. Mating pairs of red king crabs (Paralithodes
camtschaticus) in the Kodiak Archipelago, Alaska, 1960-1984. Pp. 225-246 in Paul, A. J.,
Dawe, E. G., Elner, R., Jamieson, G. S., Kruse, G. H., Otto, R. S., Sainte-Marie, B., Shirley,
T. C., and Woodby, D. (eds). Crabs in Cold Water Regions: Biology, Management, and
Economics. Alaska Sea Grant College Program AK-SG-02-01, Anchorage, USA.
Quinn II, T. J., Shirley, T. C., and Koeneman, T. M. 2006. Southeast Alaska red king crab stock
assessment review. Special Publication No. 06-12. Alaska Department of Fish and Game,
Division of Sport Fish, Research and Technical Services, Anchorage, USA. 40 p. Available:
https://www.adfg.alaska.gov/FedAidPDFs/sp06-12.pdf

32
R Core Team. 2023. R: a language and environment for statistical computing. R Foundation for
Statistical Computing, Vienna, Austria.
Saint-Marie, B., and Lovrich, G. A. 1994. Delivery and storage of sperm at first mating of
female Chionoecetes opilio (Brachyura: Majidae) in relation to size and morphometric
maturity of male parent. Journal of Crustacean Biology 14(3):508-521. DOI:
10.2307/15498997
Sharp, D. M. T., and Hendry, A. P. 2009. Life history changes in commercially exploited fish
stocks: an analysis of trends across studies. Evolutionary Applications 2(3):260-275. DOI:
10.1111/j.1752-4571.2009.00080.x
Sheilds, J. D. 2012. The impact of pathogens on exploited populations of decapod crustaceans.
Journal of Invertebrate Paleontology 110(2012):211-224. DOI: 10.1016/j.jip.2012.03.011
Sloan, L. M., and Hardy, S. M. 2017. Larval biology and environmental tolerances of the king
crab parasite Briarosaccus regalis. Journal of Parasitology 103(1):22-31. DOI: 10.1645/16-
51
Stentiford. G. D., Bateman, K. S., Feist, S. W., Oyarzún, S., Uribe, J. C., palacios, M., and Stone,
D. M. 2014. Areospora rohanae n.gen. n.sp. (Microsporidia; Areosporiidae n. fam.) elicits
multi-nucleate giant-cell formation in southern king crab (Lithodes santolla). Journal of
Invertebrate Paleontology 118:1-11. DOI: 10.1016/j.jip.2014.02.004
Stevens, B. G. 1990. Survival of king and Tanner crabs captured by commercial sale trawls.
Fishery Bulletin, U.S. 88(4):731-744.
Stevens, B. G. 2009. Hardening of red king crab (Paralithodes camtschaticus) (TILESIUS,
1815) shells after molting. Journal of Crustacean Biology 29(2):157-160. DOI: 10.1651/08-
3039.1
Stone, R. P., O’Clair, C. E., and Shirley, T. C. 1992. Seasonal migration and distribution of
female red king crabs in a southeast Alaskan estuary. Journal of Crustacean Biology
12(4):546-560. DOI: 10.1163/193724092X00030

33
Stoner, A. W., Rose, C. S., Munk, J. E., Hammond, C. F., and Davis, M. W. 2008. An
assessment of discard mortality for two Alaska crab species, Tanner crab (Chionoecetes
bairdi) and snow crab (Chionoecetes opilio), based on reflex impairment. Fishery Bulletin,
U.S. 106(4):337-347.
Urban, D., Pengilly, D., Jadamee, L., and Beyersdorfer, S. 2002. Testing carapace morphology
characteristics for the field identification of Chionoecetes hybrids. Pp. 97-113 in Paul, A. J.,
Dawe, E. G., Elner, R., Jamieson, G. S., Kruse, G. H., Otto, R. S., Sainte-Marie, B., Shirley,
T. C., and Woodby, D. (eds). Crabs in Cold Water Regions: Biology, Management, and
Economics. Alaska Sea Grant College Program AK-SG-02-01, Anchorage, USA.
U.S. Department of Commerce. 1986. Foreign Fishing; Groundfish of the Bering Sea and
Aleutian Islands. Federal Register 51(204):37407-37412. Available:
https://www.govinfo.gov/content/pkg/FR-1986-10-22/pdf/FR-1986-10-22.pdf
U.S. Department of Commerce. 1987. Groundfish of the Bering Sea and Aleutian Islands.
Federal Register 52(53):8592-8602. Available:
https://archives.federalregister.gov/issue_slice/1987/3/19/8590-8604.pdf#page=3
U.S. Department of Commerce. 1989. Foreign fishing; Groundfish of the Bering Sea and
Aleutian Islands. Federal Register 54(152):32642-32652. Available:
https://www.govinfo.gov/content/pkg/FR-1989-08-09/pdf/FR-1989-08-09.pdf
U.S. Department of Commerce. 1995a. Groundfish of the Bering Sea and Aleutian Islands Area,
North Pacific Fisheries Research Plan; trawl closure to protect red king crab. Federal
Register 60(16): 4866-4870. Available: https://www.govinfo.gov/content/pkg/FR-1995-01-
25/pdf/95-1777.pdf
U.S. Department of Commerce. 1995b. Groundfish of the Bering Sea and Aleutian Islands Area,
trawl closure to protect red king crab. Federal Register 60(237):63451-63453. Available:
https://www.govinfo.gov/content/pkg/FR-1995-12-11/pdf/95-30011.pdf
34
U.S. Department of Commerce. 1996. Fisheries of the Exclusive Economic Zone off Alaska;
groundfish of the Bering Sea and Aleutian Islands Area; trawl closure to protect red king
crab. Federal Register 61(242):65985-65989. Available:
https://www.govinfo.gov/content/pkg/FR-1996-12-16/pdf/96-31850.pdf
U.S. National Archives and Records Administration. 2023. Code of Federal Regulations, Title
50, Chapter VI, Part 679, Subpart B, § 679.22 Closures. Available:
https://www.ecfr.gov/current/title-50/chapter-VI/part-679/subpart-B/section-679.22
Yeap, A. L. K., de Souza Valente, C., Hartnett, F., Conneely, E-A., Bolton-Warburg, M., Davies,
S. J., Johnson, M. P., and Wan, A. H. L. 2022. Barriers in European spiny lobster (Palinurus
elephas) aquaculture: what we know so far? Reviews in Aquaculture 14(4):2099-2121. DOI:
10.1111/rag/12693
Yochum, N., Stoner, A. W., Sampson, D. B., and Rose, C. 2017. A comparison of laboratory-
holding and tag-return methods for evaluating delayed mortality of Dungeness crab (Cancer
magister) discarded in Oregon fisheries. Fishery Bulletin, U.S. 116:126-141. DOI:
10.7755/FB.116.1.2
Zacher, L. S., Kruse, G. H., and Hardy, S. M. 2018. Autumn distribution of Bristol Bay red king
crab using fishery logbooks. PLosOne 13(7):e0201190. DOI: 10.1371/journal.pone.0201190
Zacher, L. S., Richar, J. I., Fedewa, E. J., Ryznar, E. R., and Litzow, M. A. 2024. The 2023
eastern Bering Sea continental shelf trawl surveys: Results for commercial crab species.
U.S. Department of Commerce, NOAA Technical Memorandum NMFS-AFSC-482, 264 p.
Zheng, J., Murphy, M. C., and Kruse, G. H. 1995a. A length-based population model and stock-
recruitment relationships for red king crab, Paralithodes camtschaticus, in Bristol Bay,
Alaska. Canadian Journal of Fisheries and Aquatic Sciences 52:1229-1246. DOI:
10.1139/f95-120
Zheng, J., Murphy, M. C., and Kruse, G. H. 1995b. An update of the length-based population
model and stock-recruitment relationships for red king crab in Bristol Bay, Alaska. Alaska
Fisheries Research Bulletin 2:114-124. DOI: 10.1139/f95-120
35
Zheng, J., Murphy, M. C., and Kruse, G. H. 1997a. Alternative rebuilding strategies for the red
king crab Paralithodes camtschaticus fishery in Bristol Bay, Alaska. Journal of Shellfish
Research 16:205-217.
Zheng, J., Murphy, M. C., and Kruse, G. H. 1997b. Analysis of the harvest strategies for red king
crab, Paralithodes camtschaticus, in Bristol Bay, Alaska. Canadian Journal of Fisheries and
Aquatic Sciences 52:1229-1246. DOI: 10.1139/f95-120. Alaska Fishery Research Bulletin
54:1121-1134. DOI: 10.1139/cjfas-54-5-1121
Zheng, J., Siddeek, M. S. M., and Palof, K. J. 2021. Bristol Bay Red King Crab Stock
Assessment in Fall 2021. Discussion Paper C1 BBRKC SAFE October 2021. North Pacific
Fishery Management Council, Anchorage, USA. Available:
https://meetings.npfmc.org/CommentReview/DownloadFile?p=3ada484b-7d8c-42f0-9360-
abc1560aa669.pdf&fileName=2%20Bristol%20Bay%20Red%20King%20Crab%20SAFE.
pdf
Zhou, S., and Kruse, G. 1999. Capture efficiency and size selectivity of two types of pots for red
king crabs in the Bering Sea. Alaska Fishery Research Bulletin 6(2):94-103.

36
Table 1. -- Number of red king crab (Paralithodes camtschaticus) captured at standard survey
stations during the 2023 Bristol Bay Collaborative Pot Sampling (CPS1) survey, and
the proportion of each demographic (i.e., sex, size, or maturity category) that was
captured, within each of four trawl management areas in Bristol Bay, Alaska. For
males, mature-sized versus immature-sized simply represents a size distinction (i.e.,
based on carapace length) that follows regulatory convention, irrespective of any
individual crab’s actual reproductive status. In inches, the cutoff for legal-size is
5.3 in and for mature-size is 4.7 in. For females, maturity indicates morphometric
maturity (i.e., ability to spawn). Trawl management areas are abbreviated as follows:
NBBTCA = Nearshore Bristol Bay Trawl Closure Area; RKCSA = Red King Crab
Savings Area; RKCSS = Red King Crab Savings Subarea; BLZ1-W = waters of
Bycatch Limitation Zone 1 west of 164° 00’ W longitude and not included within the
RKCSA. See Figure 2 for the boundaries of each area. Note that the RKCSS is
subdivision within the RKCSA; therefore, the Total listed for each demographic is
smaller than the sum of catches across all areas; that is, the Total will exclude the
catch listed for the RKCSS.
NBBTCA
RKCSA
RKCSS
BLZ1-W
Demographic
Total
#
%
#
%
#
%
#
%
Legal-size (≥ 135 mm) males
3,498
2,160
61.7
689
19.7
66
1.9
649
18.6
Sublegal-size (< 135 mm) males
4,326
2,796
64.6
804
18.6
308
7.1
726
16.8
Mature-size (≥ 120 mm) males
5,000
3,098
62.0
979
19.6
122
2.4
923
18.5
Immature-size (< 120 mm) males
2,824
1,858
65.8
514
18.2
252
8.9
452
16.0
Mature females
1,934
1,466
75.8
336
17.4
74
3.8
132
6.8
Immature females
433
307
70.9
33
7.6
9
2.1
93
21.5
Total catch
10,191
6,729
66.0
1,862
18.3
457
4.5
1,600
15.7

37
Table 2. -- Summary of bycatch species (i.e., excluding red king crab, Paralithodes
camtschaticus) encountered at standard survey stations during the 2023 Bristol Bay
Collaborative Pot Sampling (CPS1) survey in Bristol Bay, Alaska, including the total
number of individuals of each species/sex that were captured and the number of
standard stations at which they were encountered.
Species name
Common name
# captured
# stations
Chionoecetes bairdi
male
Tanner crab
559
223
female
Tanner crab
11
10
Chionoecetes opilio
male
Snow crab
64
45
female
Snow crab
0
0
C. bairdi x opilio
male
Hybrid Tanner-snow crab
4
4
female
Hybrid Tanner-snow crab
0
0
Erimacrus isenbeckii
Horsehair crab
3
3
Hyas lyratus
Pacific lyre crab
8
8
Gadus chalcogrammus
Walleye pollock
2
2
Gadus macrocephalus
Pacific cod
1,728
510
Hippoglossus stenolepis
Pacific halibut
11
10
Lepidopsetta polyxystra
Northern rock sole
1
1
Limanda aspera
Yellowfin sole
2,393
420
Platichthys stellatus
Starry flounder
1
1
Myoxocephalus polyacanthocephalus
Great sculpin
78
69
Pleuronectes quadrituberculatus
Alaska plaice
1
1
Bathraja parmifera
Alaska skate
6
4
Rajidae spp.
Unidentified skates
11
9
Pycnopodia helianthoides
Sunflower seastar
12
6

38
Figure 1. -- Estimated abundance of mature-sized (≥ 120 mm (4.7 in) carapace length) and
morphometrically-mature female red king crab (Paralithodes camtschaticus) in
Bristol Bay, Alaska, from 1979 through 2023, derived from National Marine
Fisheries Service eastern Bering Sea summer trawl survey data.

39
Figure 2. -- Trawl closure and management areas in Bristol Bay, Alaska. The large area outlined
in black is the extent of the Bristol Bay red king crab (Paralithodes camtschaticus)
management unit; the area outlined in green indicates the intended coverage of the
Bristol Bay Collaborative Pot Sampling (CPS1) survey, conducted in March-April
2023.

40
Figure 3. -- Design and execution of stations included in the 2023 Bristol Bay (Alaska)
Collaborative Pot Sampling (CPS1) survey. The complete standard design included
all 694 stations depicted. Stations denoted in white were not fished due to logistical
and time constraints, and one station was omitted because the pot had not been
baited, resulting in a total of 637 stations fished. Stations fished by each vessel are
indicated in blue (fished by the FV Silver Spray) and yellow (FV Summer Bay).

41
Figure 4. -- Vessels that conducted pot fishing for red king crab (Paralithodes camtschaticus)
during the 2023 Bristol Bay (Alaska) Collaborative Pot Sampling (CPS1) survey.
Upper panel: FV Silver Spray. Lower panel: FV Summer Bay (photo credit: Cory
Lescher, Alaska Bering Sea Crabbers).

42
Figure 5. -- Pots used aboard the FV Silver Spray for capturing red king crab (Paralithodes
camtschaticus) during the 2023 Bristol Bay (Alaska) Collaborative Pot Sampling
(CPS1) survey. Pots were modified commercial traps fitted with smaller mesh and
meshed-over escape rings to enhance retention of juvenile and sublegal crab. Upper
panel: Side view of a pot (photo credit: Vicki Vanek, ADF&G). Note the upward-
sloping funnels and centrally-hung bait bag. Lower panel: A pot being emptied of its
catch (photo credit: Cory Lescher, Alaska Bering Sea Crabbers). Note the double-
frame construction of the pots. The black plastic hoods attached to the funnel-ends
have been propped open to become disabled; the rectangles inward of the open
hoods are the openings of the funnels into the pot. An open hood can also be seen on
the left-hand funnel in the upper image.

43
Figure 6. -- An RBR Ltd. XR-420 CTD datalogger affixed to the roof of a crab-survey pot used
to capture red king crab (Paralithodes camtschaticus) during the 2023 Bristol Bay
(Alaska) Collaborative Pot Sampling (CPS1) survey (photo credit: Vicki Vanek,
ADF&G). CTD = Conductivity (i.e., an indicator of salinity), Temperature, and
Depth meter.

44
Figure 7. -- Measurement of carapace length (CL) in red king crab (Paralithodes camtschaticus).
Upper: CL is the distance from the center of the posterior margin of the crab’s
carapace, measured from between the marginal spines, to the deepest point of one of
the crab’s eye orbits, at the base of the rostrum. Lower: a crab being measured using
digital calipers (photo credit: Cory Lescher, Alaska Bering Sea Crabbers). Note the
placement of the calipers in the crab’s right eye orbit (the left orbit is visible on the
near side of the rostrum).

45
Figure 8. -- A Bristol Bay red king crab (Paralithodes camtchaticus) tagged with a Wildlife
Computers (Redmond, Washington, USA) Pop-up Archival Transmitting (PAT)
during the 2023 Bristol Bay (Alaska) Collaborative Pot Sampling (CPS1) survey
(photo credit: Cory Lescher, Alaska Bering Sea Crabbers). The tag is attached to a
polyolefin tubing harness that wraps around the crab’s carapace.

46
Figure 9. -- Distribution of soak times employed during the 2023 Bristol Bay (Alaska)
Collaborative Pot Sampling (CPS1) survey, targeting red king crab (Paralithodes
camtschaticus).

47
Figure 10. --Bottom temperature in the CPS1 area. Top panels: Summer temperatures from the
NMFS survey. Bottom panel: March/April temperature from CPS1, with logger
locations.

48
Figure 11. --Sea ice conditions in Bristol Bay (Alaska) during the course of the 2023 Bristol Bay
(Alaska) Collaborative Pot Sampling (CPS1) survey. Ice cover ranges in density
from red (nearly complete coverage) to light blue (open water with < 10% ice). Sea
ice was not encountered at any of the survey stations but occurred just north of the
survey grid at the initiation of sampling.

49
Figure 12. --Lower panel: size frequency (by carapace length) distribution of all male red king
crab (Paralithodes camtschaticus) captured during the 2023 Bristol Bay (Alaska)
Collaborative Pot Sampling (CPS1) survey, depicting the abundance of individuals
within 1 mm size bins possessing various shell conditions. Upper panels: male shell
conditions, by length, as observed during U.S. National Marine Fisheries Service
trawl survey data within the Bristol Bay District during the summers of 2022 and
2023.

50
Figure 13. -- Lower panel: size frequency (by carapace length) distribution of all female red
king crab (Paralithodes camtschaticus) captured during the 2023 Bristol Bay
(Alaska) Collaborative Pot Sampling (CPS1) survey, depicting the abundance
within 1 mm size bins of morphometrically mature and immature individuals.
Upper panels: female maturity, by length, as observed during U.S. National
Marine Fisheries Service trawl survey data within the Bristol Bay District during
the summers of 2022 and 2023.

51
Figure 14. -- Lower panel: size frequency (by carapace length) distribution of
morphometrically-mature female red king crab (Paralithodes camtschaticus)
captured during the 2023 Bristol Bay (Alaska) Collaborative Pot Sampling (CPS1)
survey, depicting the abundance of individuals within 1 mm size bins possessing
various shell conditions. Upper panels: mature female shell conditions, by length,
as observed during U.S. National Marine Fisheries Service trawl survey data
within the Bristol Bay District during the summers of 2022 and 2023.

52
Figure 15. -- Lower panel: size frequency (by carapace length) distribution of morphometrically
mature female red king crab (Paralithodes camtschaticus) captured bins during the
2023 Bristol Bay (Alaska) Collaborative Pot Sampling (CPS1) survey, depicting
the abundance of individuals within 1 mm size bins exhibiting each measure of egg
condition. Upper panels: mature female egg conditions, by length, as observed
during U.S. National Marine Fisheries Service trawl survey data within the Bristol
Bay District during the summers of 2022 and 2023.

53
Figure 16. -- Lower panel: size frequency (by carapace length) distribution of morphometrically
mature female red king crab (Paralithodes camtschaticus) captured during the
2023 Bristol Bay (Alaska) Collaborative Pot Sampling (CPS1) survey, depicting
the abundance of individuals within 1 mm size bins displaying each measure of
clutch fullness condition. Upper panels: mature female egg conditions, by length,
as observed during U.S. National Marine Fisheries Service trawl survey data
within the Bristol Bay District during the summers of 2022 and 2023.

54
Figure 17. --Spatial distribution and relative abundance of all red king crab (Paralithodes
camtschaticus) captured during the 2023 Bristol Bay (Alaska) Collaborative Pot
Sampling (CPS1) survey. Spot size is proportional to the number of crabs captured
at each location, as indicated in the legend.

55
Figure 18. -- Lower panel: Spatial distribution and relative abundance of male red king crab
(Paralithodes camtschaticus) ≥ 135 mm (5.3 in) carapace length (i.e., legal-size)
captured during the 2023 Bristol Bay (Alaska) Collaborative Pot Sampling (CPS1)
survey. Spot size is proportional to the number of crabs captured at each location,
as indicated in the legend. Abundance data are overlain on smoothed bottom
temperatures obtained from temperature loggers placed inside the pots. Upper
panels: legal-size male red king crab abundance and bottom temperatures observed
during U.S. National Marine Fisheries Service trawl survey data within the Bristol
Bay District during the summers of 2022 and 2023.

56
Figure 19. -- Lower panel: Spatial distribution and relative abundance of male red king crab
(Paralithodes camtschaticus) ≥120 mm (4.7 in) carapace length (i.e., mature-size)
captured during the 2023 Bristol Bay (Alaska) Collaborative Pot Sampling (CPS1)
survey. Spot size is proportional to the number of crabs captured at each location,
as indicated in the legend. Abundance data are overlain on smoothed bottom
temperatures obtained from temperature loggers placed inside the pots. Upper
panels: mature-size male red king crab abundance and bottom temperatures
observed during U.S. National Marine Fisheries Service trawl survey data within
the Bristol Bay District during the summers of 2022 and 2023.
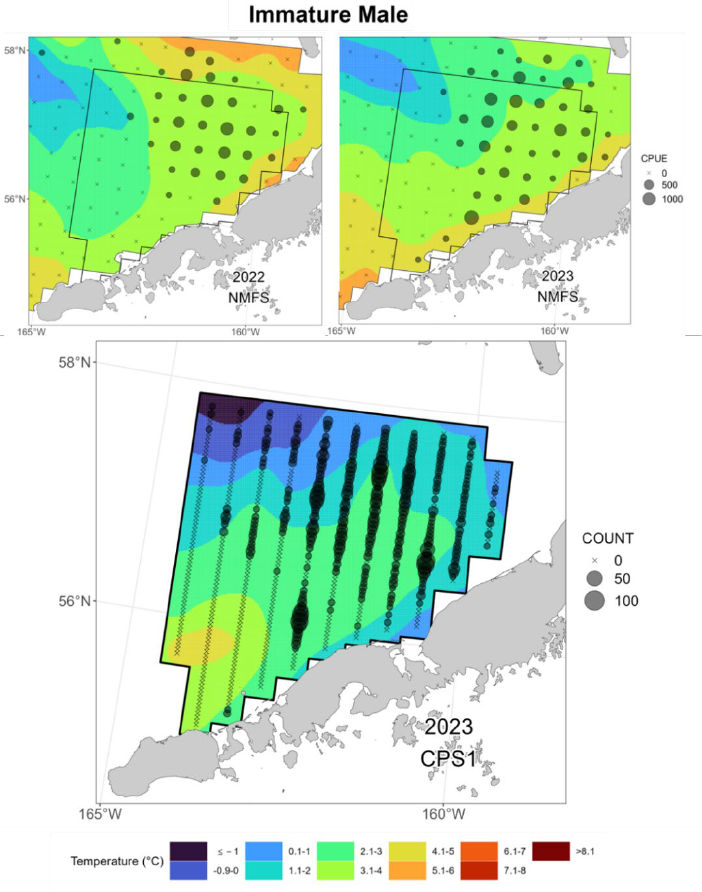
57
Figure 20. -- Lower panel: Spatial distribution and relative abundance of male red king crab
(Paralithodes camtschaticus) < 120 mm (4.7 in) carapace length (i.e., immature-
size) captured during the 2023 Bristol Bay (Alaska) Collaborative Pot Sampling
(CPS1) survey. Spot size is proportional to the number of crabs captured at each
location, as indicated in the legend. Abundance data are overlain on smoothed
bottom temperatures obtained from temperature loggers placed inside the pots.
Upper panels: immature-size male red king crab abundance and bottom
temperatures observed during U.S. National Marine Fisheries Service trawl survey
data within the Bristol Bay District during the summers of 2022 and 2023.
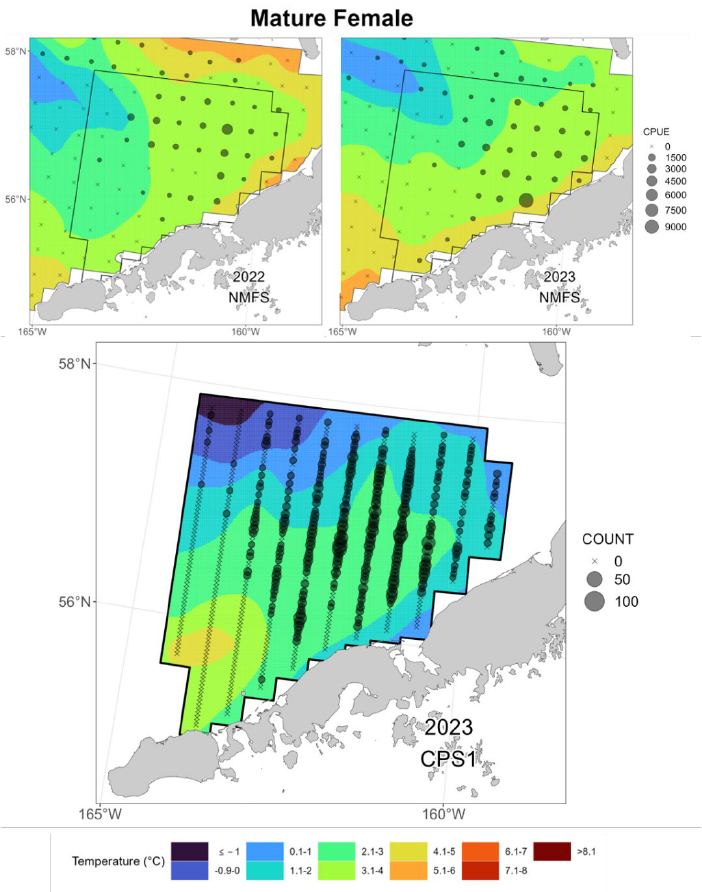
58
Figure 21. -- Lower panel: Spatial distribution and relative abundance of morphometrically
mature female red king crab (Paralithodes camtschaticus) captured during the
2023 Bristol Bay (Alaska) Collaborative Pot Sampling (CPS1) survey. Spot size is
proportional to the number of crabs captured at each location, as indicated in the
legend. Abundance data are overlain on smoothed bottom temperatures obtained
from temperature loggers placed inside the pots. Upper panels: mature female red
king crab abundance and bottom temperatures observed during U.S. National
Marine Fisheries Service trawl survey data within the Bristol Bay District during
the summers of 2022 and 2023.

59
Figure 22. -- Lower panel: Spatial distribution and relative abundance of morphometrically
immature female red king crab (Paralithodes camtschaticus) captured during the
2023 Bristol Bay (Alaska) Collaborative Pot Sampling (CPS1) survey. Spot size is
proportional to the number of crabs captured at each location, as indicated in the
legend. Abundance data are overlain on smoothed bottom temperatures obtained
from temperature loggers placed inside the pots. Upper panels: immature female
red king crab abundance and bottom temperatures observed during U.S. National
Marine Fisheries Service trawl survey data within the Bristol Bay District during
the summers of 2022 and 2023.

60
Figure 23. --Spatial distribution and relative abundance of all Tanner crab (Chionoecetes bairdi)
captured during the 2023 Bristol Bay (Alaska) Collaborative Pot Sampling (CPS1)
survey. Spot size is proportional to the number of crabs captured at each location, as
indicated in the legend. Abundance data are overlain on smoothed bottom
temperatures obtained from temperature loggers placed inside the pots.

61
Figure 24. --Spatial distribution and relative abundance of all snow crab (Chionoecetes opilio)
captured during the 2023 Bristol Bay (Alaska) Collaborative Pot Sampling (CPS1)
survey. Spot size is proportional to the number of crabs captured at each location, as
indicated in the legend. Abundance data are overlain on smoothed bottom
temperatures obtained from temperature loggers placed inside the pots.

62
Figure 25. --Spatial distribution and relative abundance of all Pacific cod (Gadus
macrocephalus) captured during the 2023 Bristol Bay (Alaska) Collaborative Pot
Sampling (CPS1) survey. Spot size is proportional to the number of fish captured at
each location, as indicated in the legend. Abundance data are overlain on smoothed
bottom temperatures obtained from temperature loggers placed inside the pots.

63
Figure 26. --Spatial distribution and relative abundance of all yellowfin sole (Limanda aspera)
captured during the 2023 Bristol Bay (Alaska) Collaborative Pot Sampling (CPS1)
survey. Spot size is proportional to the number of fish captured at each location, as
indicated in the legend. Abundance data are overlain on smoothed bottom
temperatures obtained from temperature loggers placed inside the pots.

64
Figure 27. --Movement vectors for new hard-shell, mature-size male Bristol Bay red king crab
(Paralithodes camtschaticus) that were tagged with Pop-up Archival Transmitting
(PAT) tags in late March to early April 2023, during the 2023 Bristol Bay (Alaska)
Collaborative Pot Sampling (CPS1) survey, and released from crab and reported
locations via satellite from 1 to 3 June 2023.

65
Figure 28. -- Rose plot showing movement rate and direction of movement between tagging and
tag reporting for new hard-shell, mature-size male Bristol Bay red king crab
(Paralithodes camtschaticus) that were tagged with Pop-up Archival Transmitting
(PAT) tags in late March to early April 2023, during the 2023 Bristol Bay (Alaska)
Collaborative Pot Sampling (CPS1) survey, and whose tags released and reported
final locations via satellite from 1 to 3 June 2023.

66
Figure 29. --Pop-up locations for pop-up archival transmitting (PAT) tags that were placed on
new hard-shell mature Bristol Bay red king crab (Paralithodes camtschaticus)
during the 2023 Bristol Bay (Alaska) Collaborative Pot Sampling (CPS1) survey.
Tags surfaced while the NMFS eastern Bering Sea trawl survey was surveying
Bristol Bay. Pop-up locations are overlaid on trawl survey results for new hard-shell
mature-size males.

U.S. Secretary of Commerce
Gina M. Raimondo
Under Secretary of Commerce for
Oceans and Atmosphere
Dr. Richard W. Spinrad
Assistant Administrator, National Marine
Fisheries Service.
Janet Coit
April 2024
www.nmfs.noaa.gov
OFFICIAL BUSINESS
National Marine
Fisheries Service
Alaska Fisheries Science Center
7600 Sand Point Way N.E.
Seattle, WA 98115-6349
