
Kunruedee Sangseethong
a
Sirithorn Lertphanich
b
Klanarong Sriroth
b, c
a
Cassava and
Starch Technology
Research Unit, National Center
for Genetic Engineering
and Biotechnology,
Bangkok, Thailand
b
Department of
Biotechnology, Faculty
of Agro-Industry,
Kasetsart University,
Bangkok, Thailand
c
Kasetsart Agricultural and
Agro-Industrial Product
Improvement Institute,
Kasetsart University,
Bangkok, Thailand
Physicochemical Properties of Oxidized Cassava
Starch Prepared under Various Alkalinity Levels
The physicochemical properties of hypochlorite-oxidized cassava starch as influenced
by the alkalinity levels (pH 8 to 11) during modification process were investigated.
Hypochlorite oxidation generally increased the contents of carbonyl and carboxyl
groups in starch but decreased starch viscosity. The formation of carbonyl and car-
boxyl groups was more favorable under the milder alkaline conditions (pH 8 and 9).
Oxidation conducted at higher alkalinity levels produced both functional groups at a
much slower rate and to a lesser extent. Starch viscosity decreased markedly with
increasing reaction time. The alkalinity levels during the modification process greatly
influenced the initial viscosity of the oxidized starch paste and the viscosity stability of
the paste during storage. Thermal behavior studies by differential scanning calorimetry
(DSC) demonstrated that oxidation decreased both gelatinization temperature and
enthalpy. The decrease in gelatinization temperature was strongly related to the car-
boxyl group content. The more carboxyl groups the oxidized starch contained, the
lower was the gelatinization temperature. Retrogradation of amylopectin tended to
increase slightly after oxidation. While the light transmittance of native starch paste
drastically decreased during cold storage, the changes observed in oxidized starch
pastes were less pronounced and appeared to depend on carboxyl content. The
results from light transmittance studies suggested that carboxyl groups introduced
into the starch molecules could effectively prevent retrogradation.
Keywords: Alkalinity; Cassava starch; Hypochlorite; Oxidized starch; Physicochem-
ical properties
92 Starch/Stärke 61 (2009) 92–100
1 Introduction
Oxidized starch is widely used in many industries, partic-
ularly in applications where film formation and adhesion
properties are desired. The major application of oxidized
starch is as surface sizing agent and coating binder in the
paper industry. Oxidized starch is commonly produced by
reaction of starch with an oxidizing agent under controlled
temperature and pH. Although many oxidizing agents are
available, hypochlorite is the most commonly used
chemical in the commercial production of oxidized starch.
During the oxidation of starch, different reactions occur,
leading to the introduction of carbonyl and carboxyl
groups, and to the degradation of the starch molecules.
The desired properties of oxidized starch are mainly lower
viscosity and improved starch paste stability.
Several investigations on oxidation of amylose, amylo-
pectin and native starches from various origins have been
reported [1-5]. It has been claimed that oxidation occur-
red mainly in the amorphous regions, because no change
in the X-ray patterns and intensity was observed in the
oxidized products [4, 6]. Hypochlorite oxidation has been
reported to depend mainly on the pH during the reaction.
Whistler and Schweiger [2] demonstrated that hypo-
chlorite oxidation of corn amylopectin was most rapid at
neutral pH while the reaction rate decreased with
increasing acidity and alkalinity. Similar results were
observed on wheat and corn starches [7, 8]. The type and
amount of functional groups formed in the starch mole-
cules depend on the reaction pH as well. The formation of
carbonyl groups was found to be higher under acidic
conditions while the amount of carboxyl groups increased
with increasing pH [6, 9].
Beside the reaction pH, other factors such as oxidant
concentration, temperature and starch origin are known
to influence hypochlorite oxidation. It has been shown
that different levels of sodium hypochlorite used in the
reaction yielded oxidized products with different pasting
properties. The slightly oxidized starch exhibited a past-
ing profile similar to that of slightly crosslinked starch
while at high degree of oxidation the starch had a lower
viscosity which is a typical characteristic of oxidized
Correspondence: Kunruedee Sangseethong, Cassava and
Starch Technology Research Unit - National Center for Genetic
Engineering and Biotechnology (BIOTEC) Kasetsart University,
Jatujak Bangkok 10900, Thailand. Phone: 166-2940 5634, Fax:
© 2009 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.starch-journal.com
Research Paper
DOI 10.1002/star.200800048
Starch/Stärke 61 (2009) 92–100 Physicochemical Properties of Oxidized Cassava Starch 93
starch [10, 11]. The botanical origin of starch used in the
modification process is of considerable importance to the
course of reaction and the properties of the modified
starch. Tuber starches were reported to be more readily
oxidized than cereal starches [3, 4]. Amylose content of
native starch was also suggested to play an important
role in controlling the oxidation efficiency [5].
Currently, for industrial practice, hypochlorite oxidation of
starch is performed under mildly to moderately alkaline
conditions in order to favor the production of carboxyl
groups, which are a key component in stabilizing the vis-
cosity of starch dispersion and minimizing retrogradation
[12]. A range of reaction pH from 8 to 11 can be encoun-
tered in different starch modification plants. As mentioned
above, reaction pH is a key factor in determining the
course of hypochlorite oxidation resulting in different
reaction rates and products with different chemical
structures. Although several studies have been carried
out to demonstrate the influence of reaction pH on hypo-
chlorite oxidation, most of them were performed under
more drastic conditions (at very low starch concentration
and relatively high levels of oxidant) than those normally
used in the industrial practice. Furthermore, the studies
on reaction rate were performed based on the rate of
hypochlorite disappearance which might not be relevant
to the changes occurring to the starch molecules. The
products determined in these studies corresponded to a
relatively high degree of oxidation, which might not
represent what really takes place in the commercial
products.
Relatively few reports are available in the literature on the
physicochemical and functional properties of oxidized
starch as influenced by reaction pH. The aim of this study
was to determine and compare the physicochemical
properties of hypochlorite oxidized cassava starch pre-
pared under different levels of alkalinity. The results from
this study could provide information used to improve the
manufacturing process as well as the starch properties for
certain applications.
2 Materials and Methods
2.1 Materials
Native cassava starch was obtained from Sanguan
Wongse Industries Co., Ltd., Nakhonratchasima, Thai-
land. Sodium hypochlorite containing 10% (w/w) active
chlorine was obtained from B.S. International Co., Ltd.
(Bangkok, Thailand). All other chemicals used in the study
were of analytical grade.
2.2 Preparation of oxidized starch
A cassava starch slurry containing 40% dry solids was
prepared and the pH was adjusted to 8, 9, 10, and 11 with
aqueous NaOH solution. The temperature of the slurry
was adjusted to 307C and sodium hypochlorite (3% active
chlorine based on starch) was added dropwise over a
period of 15 min with stirring. During the addition of
reagent and the course of reaction, the pH of the slurry
was maintained at the desired value with NaOH or HCl
solution. The mixture was stirred under the defined con-
ditions and samples were collected at 30, 60, 120 and 300
min. The reaction in the collected samples was termi-
nated by addition of sodium bisulfite and the pH was
adjusted to 6.5-7.0. The samples were then filtered and
thoroughly washed with water until the filtrate gave
negative response to silver nitrate solution. The obtained
starch was then dried in an oven at 507C.
2.3 Determination of carbonyl content
The carbonyl content was determined as described by
Kuakpetoon and Wang [4]. A starch sample (4 g) was
slurried in 100 mL of distilled water. The slurry was gelati-
nized in a boiling water bath for 20 min, cooled to 407C
and adjusted to pH 3.2 with 0.1 M aqueous HCl. Then 15
mL of hydroxylamine reagent was added. The flask was
stoppered and agitated in a water bath at 407C. After 4 h,
the pH of the sample was rapidly brought to 3.2 with 0.1 M
aqueous HCl. A blank determination with only hydroxyl-
amine reagent was performed in the same manner. The
hydroxylamine reagent was prepared by dissolving 25 g
hydroxylamine hydrochloride in 100 mL of 0.5 M aqueous
NaOH. The final volume was then adjusted to 500 mL with
distilled water.
2.4 Determination of carboxyl content
The carboxyl content of starch was determined following
the FAO method [13] with some modification. A starch
sample (5 g) was stirred in 25 mL 0.1 M aqueous HCl for
30 min. The slurry was then filtered and washed with dis-
tilled water until free of chloride ions. The filtered cake
was transferred to a 600 mL beaker, and the volume was
adjusted to 300 mL with distilled water. The starch slurry
was heated in a boiling water bath with continuous stirring
for 15 min to ensure complete gelatinization. The hot
sample was immediately titrated with 0.1 M aqueous
NaOH using phenolphthalein as indicator. A blank deter-
mination was run on the original sample in the same
manner. In the blank determination the sample had pre-
viously been stirred in 25 mL distilled water instead of 0.1
M HCl.
© 2009 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.starch-journal.com

94 K. Sangseethong et al. Starch/Stärke 61 (2009) 92–100
2.5 Viscosity and viscosity stability of starch
dispersion
The viscosity and viscosity stability of oxidized starch
pastes were deterimined on a rotational Physica MCR 300
rheometer (Physica Messtechnik GmbH, Stuttgart, Ger-
many) using a concentric cylinder (diameter of cup and
bob, 28.92 and 26.66 mm, respectively). The temperature
was regulated by a Paar Physica circulating bath and a
controlled Peltier system (TEZ 150P/MCR) with an accu-
racy of
1 0.17C. Thestarch paste was prepared by heating
15%(w/w) of starchslurryin a water bath at 957Cfor 15 min
with constant stirring at 200 rpm. During heating, the
sample jar was covered with a lid to avoid water loss. The
hot paste was immediately transferred to the sample cup
inwhich the temperature was pre-set at 507C. The bob was
then lowered to the measurement position and the sample
was allowed to equilibrate at the pre-set temperature.
When the temperature of the starch paste reached 507C,
the initial viscosity of the fresh sample was measured over
a shear rate range of 0.1–500 s
-1
. The viscosity values at
the shear rate of 22 s
-1
were used for comparison between
different samples. The viscosity stability of the starch
paste was determined by measuring the viscosity of the
sample after being maintained at 507C for 8 h.
2.6 Gelatinization and retrogradation properties
by DSC
The gelatinization and retrogradation properties of the
starch samples were measured using a Perkin-Elmer Dif-
ferential Scanning Calorimeter (DSC7, Norwalk, CT).
Starch was weighed into a stainless steel DSC pan and
deionized water was added to give 70% moisture con-
tent. The pan was sealed, equilibrated at room tempera-
ture overnight, and scanned from 0 to 1207C at a rate of
107C/min. After scanning, the gelatinized sample was
stored at 47C for seven days, after which the sample was
left at room temperature for 1 h and rescanned under the
same conditions with the first scanning. An empty pan
was used as the reference and the DSC was calibrated
with indium. The onset (T
o
), peak (T
p
) and conclusion (T
c
)
temperatures and the enthalpies of gelatinization (DH
g
)
and retrogradation (DH
r
) were determined.
2.7 Light transmittance of starch paste
Light transmittance of the starch paste was measured as
described by Jacobson et al. [14] with some modification.
A 5% (w/w) aqueous suspension of starch in a screw-
capped test tube was heated in a boiling water bath for 30
min with constant shaking for the first 5 min and occa-
sional shaking afterward. The sample was then cooled at
room temperature for 30 min. The initial transmittance of
the fresh sample was determined at 650 nm against water
blank using a Spectronic Genesys-5 (Milton Roy Com-
pany, New York, USA). Samples were also stored at 47Cin
a refrigerator, to monitor a tendency for retrogradation,
and the transmittance of samples was determined during
cold storage for up to seven days.
3 Results and Discussion
3.1 Carbonyl and carboxyl contents
The carbonyl and carboxyl contents of oxidized cassava
starches as a function of reaction pH and time are shown
in Figs. 1 and 2. All oxidized samples contained higher
amounts of carboxyl than carbonyl groups which is in
accordance with the earlier works reporting that hypo-
chlorite oxidation of starch under alkaline conditions
favored the formation of carboxyl group [12]. The alkali-
nity level during modification process had a significant
influence on the type and amount of functional groups
formed in the oxidized starch. Within the pH range stud-
ied, the amount of carbonyl groups was highest at pH 8
and decreased as the reaction pH increased, which is in
agreement with previous reports [6, 9]. Irrespective of
reaction pH, the rate of carbonyl group formation was
fast. After 30 min of reaction time, no change in carbonyl
content was observed (Fig. 1).
As shown in Fig. 2, the highest formation of carboxyl
groups was observed when the oxidation was conducted
under mildly alkaline conditions (pH 8 and 9). Under these
conditions, the rate of carboxyl group formation was also
fast; carboxyl content remained constant after only 30
min of reaction time. As the reaction pH increased to 10
and 11, the amount of carboxyl groups and its formation
rate decreased. The present result differs from earlier
studies [6, 9] which reported that the formation of car-
boxyl group was more favorable at higher reaction pH.
This discrepancy might be due to different conditions
employed in the reactions. In earlier studies, more drastic
conditions with much longer reaction times were used.
The milder conditions and the range of reaction times
used in the current study were more relevant to the
industrial practice; thus, the results obtained could be
pertinent to the industrial applications.
3.2 Viscosity and viscosity stability of starch
paste
The apparent viscosity of oxidized starch was measured
in a 15% suspension and at 507C. Under these condi-
tions, the paste from native cassava starch was too vis-
© 2009 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.starch-journal.com

Starch/Stärke 61 (2009) 92–100 Physicochemical Properties of Oxidized Cassava Starch 95
Fig. 1. Carbonyl content of
native and oxidized cassava
starches prepared under dif-
ferent reaction pH and
times. Native (
), pH 8 ( ),
pH 9 (
), pH 10 ( ), and pH
11 (
).
Fig. 2. Carboxyl content of
native and oxidized cassava
starches prepared under dif-
ferent reaction pH and
times. Native (
), pH 8 ( ),
pH 9 (
), pH 10 ( ), and pH
11 (
).
cous to measure; hence its viscosity was not deter-
mined. The effects of reaction pH and time on the
initial viscosity and viscosity stability of the starch
paste (as demonstrated by the changes in the paste
viscosity after 8 h storage) are presented in Tab. 1. As
noted from the fresh pastes, the viscosity of oxidized
starches progressively decreased with increasing
reaction time. This decrease was presumably caused
by partial cleavage of the glucosidic linkages upon
oxidation, resulting in a decrease in molecular weight
of starch molecules [4, 15]. The rate and extent of
viscosity reduction varied depending on the alkalinity
levels during modification process. Modification under
the highest alkalinity level (i.e. pH 11) yielded oxidized
starch with the lowest paste viscosity. In addition to
oxidative cleavage, alkaline degradation could con-
tribute to the higher degree of viscosity reduction
observed for oxidized starch modified under strongly
alkaline conditions [16].
Improved stability of starch dispersions is one of the key
characteristics desired from oxidized starch. It has been
suggested that carboxyl groups introduced to starch
molecules would hinder the chain re-association or retro-
gradation; hence improving the viscosity stability of the
starch paste [12]. In this study, the viscosity of starch
paste after being maintained at 507C for 8 h was deter-
mined and compared with the viscosity of the fresh paste
(Tab. 1). The data indicated that reaction pH and time
significantly influenced the stability of the starch paste.
Two different characteristics were observed for oxidized
starches modified under various reaction pHs. Starch
© 2009 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.starch-journal.com

96 K. Sangseethong et al. Starch/Stärke 61 (2009) 92–100
Tab. 1. Viscosity (mPa s) of fresh and stored (8 h at 507C)
oxidized cassava starch pastes (15% solid)
measured at a shear rate of 22 s
-1
and a temper-
ature of 507C.
Reaction
pH
Reaction
time [min]
Storage time [h]
0
(fresh paste)
8
(stored paste)
8 30 3570
1 124 302 1 7.2
60 1430
1 184 109 1 11
120 64
1 5.9 49 1 2.0
300 31
1 1.7 29 1 1.1
9 30 3965 1 134 2870 1 184
60 2460
1 353 1660 1 138
120 1370
1 175 445 1 33
300 70
1 6.8 65 1 4.7
10 30 3250
1 200 4030 1 330
60 122
1 7.5 313 1 29
120 46
1 1.7 57 1 2.1
300 30
1 2.3 34 1 1.8
11 30 401
1 29 4360 1 328
60 44
1 2.5 121 1 14
120 19
1 1.3 23 1 0.7
300 15
1 0.1 17 1 1.3
modified under the milder alkaline conditions (pH 8 and 9)
exhibited a decrease in paste viscosity during storage. In
contrast, starch modified at higher alkalinity (pH 10 and
11) showed an increase in viscosity after storage. Irre-
spective of reaction pH, the changes in paste viscosity
were prominent for starch modified with shorter reaction
time. The stability of the viscosity of the starch paste was
much improved when the oxidation was performed at
longer reaction time (300 min).
It might be expected that the reduction in the viscosity of
the stored paste observed for oxidized starch modified at
pH 8 and 9 was attributed to depolymerization of the
starch molecules. The fact that this behavior was not
observed for the samples modified at higher pH values
implied that hypochlorite oxidation under different alkali-
nity levels would yield oxidized products with different
molecular structures. Modification at the milder alkalinity
levels might lead to oxidized starch with more labile
structures that are sensitive to degradation or hydrolysis,
causing a viscosity decrease of starch paste during stor-
age. Prey and Siklossy [17] observed a similar phenom-
enon. In their study, although the oxidized starch was kept
in a dry powder form, after one year a substantial reduc-
tion in starch viscosity was noted. They related this
observation to the presence of aldehyde/carbonyl
groups. Starch with higher aldehyde/carbonyl content
exhibited a larger degree of viscosity reduction. In our
current study, similar results were observed. Starch
modified under more mildly alkaline conditions, which
contained a higher fraction of carbonyl groups (Fig. 1),
exhibited a high degree of viscosity reduction.
For starch modified at the higher alkalinity levels (pH 10
and 11), the paste viscosity significantly increased after
storage. The increase in viscosity of the stored paste
observed in this group of samples could be due to the
intermolecular re-association or retrogradation of the
starch molecules. The extent was more pronounced for
starch oxidized with a shorter reaction time, indicating
that starch with low degree of oxidation had a higher ten-
dency for retrogradation. As the degree of oxidation
increased (i.e. samples with longer reaction time), the
paste viscosity seemed to be more stable. The marked
decrease in molecular size and the substantial increase in
carboxyl content were likely responsible for the lower
tendency for molecular re-association in these samples.
3.3 Gelatinization properties
The gelatinization properties of native and oxidized cas-
sava starches measured by DSC are summarized in Tab.
2. In general, the onset of gelatinization temperature (T
o
)
and the gelatinization enthalpy (DH
g
) of the oxidized star-
ches were lower than those of native starch, and the
extent of changes varied with modification conditions.
Many studies have reported the influence of oxidation on
the gelatinization properties of starch but the results are
somewhat inconclusive and seem to depend on starch
origin as well as the modification conditions. Forssell et al.
[3] observed an increase in T
o
of oxidized barley starch,
while the T
o
of oxidized potato starch remained unchan-
ged. Wang and Wang [11] reported an increase in T
o
of
oxidized waxy and common corn starches, when a low
concentration of hypochlorite (
, 1.25% active chlorine)
was used in the modification process. However, when the
oxidant concentration was increased (up to 5% active
chlorine), a decrease in T
o
of oxidized starches was noted
[5]. In our current study, a decrease in T
o
of oxidized cas-
sava starches was observed, which could be attributed to
the introduction of carboxyl groups into the starch mole-
cules. It is expected that the carboxyl group carrying a
negative charge could readily adsorb water and facilitate
hydration, thus causing an increase in the swelling of
starch granules and a decrease in the gelatinization tem-
perature.
The extent of decrease in T
o
of the oxidized starch (as
shown in Tab. 2) was strongly related to the carboxyl
group content in the starch sample (Fig. 2). Oxidized
starch produced under the milder alkaline conditions (pH
8 and 9), which contained a higher amount of carboxyl
© 2009 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.starch-journal.com
Starch/Stärke 61 (2009) 92–100 Physicochemical Properties of Oxidized Cassava Starch 97
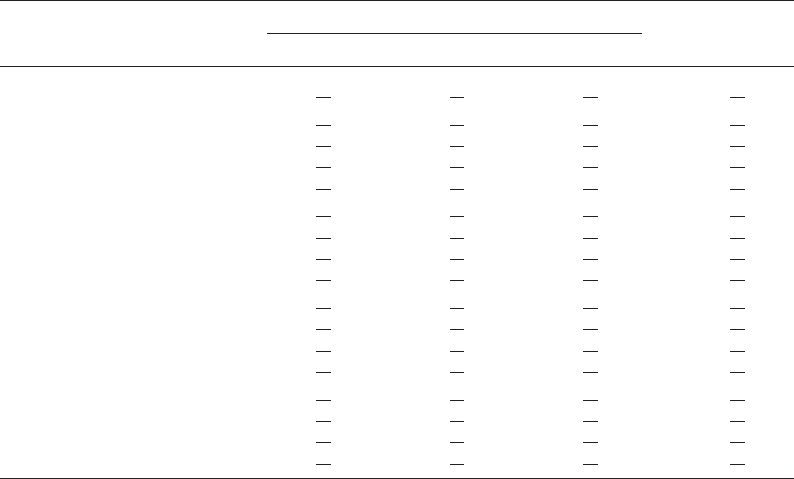
Tab. 2. Gelatinization properties of native and oxidized cassava starches.
Reaction pH Reaction
time [min]
Transition temperature [7C] Gelatinization
enthalpy [J/g]
T
o
T
p
T
c
Native - 64.26 1 0.02 71.70 1 0.00 81.00 1 0.02 17.40 1 0.13
8 30 60.05
1 0.07 67.40 1 0.00 90.74 1 0.03 15.33 1 0.24
60 60.52
1 0.04 68.15 1 0.49 88.24 1 1.05 15.37 1 0.34
120 61.34
1 0.57 68.95 1 0.92 89.37 1 0.21 14.28 1 1.15
300 60.68
1 0.19 68.45 1 0.21 90.48 1 0.47 15.14 1 0.04
9 30 59.84
1 0.73 67.70 1 0.99 81.42 1 0.32 15.74 1 0.24
60 59.43
1 0.43 66.90 1 0.85 87.53 1 0.40 15.55 1 0.54
120 58.60
1 0.08 65.70 1 0.14 88.47 1 0.33 15.05 1 0.05
300 59.63
1 0.18 67.20 1 0.57 89.12 1 0.01 14.92 1 0.02
10 30 62.94
1 0.16 70.85 1 0.21 85.36 1 0.96 16.56 1 0.22
60 61.94
1 0.31 68.85 1 0.35 88.28 1 0.37 15.94 1 0.43
120 61.73
1 0.37 69.20 1 0.42 88.83 1 0.25 15.63 1 0.17
300 61.59
1 0.18 68.90 1 0.00 89.92 1 0.41 14.14 1 0.03
11 30 64.46
1 0.16 72.25 1 0.07 86.64 1 1.77 16.14 1 0.33
60 64.08
1 0.19 71.65 1 0.64 89.44 1 0.18 15.49 1 0.28
120 63.54
1 0.08 71.15 1 0.35 91.50 1 0.21 14.20 1 0.05
300 63.08
1 0.22 70.70 1 0.71 91.93 1 0.64 14.40 1 0.50
groups exhibited a greater reduction in T
o
. On the other
hand, starch modified at higher pH containing a lower
amount of carboxyl groups showed a smaller decrease in
T
o
. Although carboxyl content markedly correlated to the
changes in T
o
of oxidized starch, this factor alone cannot
completely explain the differences in T
o
between some of
the oxidized samples. For instance, starch oxidized at pH
11 with reaction times longer than 60 min had a higher
carboxyl content but exhibited a smaller reduction in T
o
when compared to oxidized starch obtained at pH 10 with
a reaction time of 30 min. This suggests that other factors
such as the distribution or location of functional groups
and the degradation pattern of starch molecules might
also play important roles in determining the gelatinization
properties of oxidized starch [5, 18].
The gelatinization enthalpies (DH
g
) of oxidized starches
were lower than that of native starch. The decrease of D H
g
indicated that oxidation caused a weakening of the starch
granules, probably from the partial degradation of starch
molecules in the crystalline lamellae. Consequently, less
energy was needed to gelatinize starch.
3.4 Retrogradation properties
The retrogradation properties of native and oxidized star-
ches after storage at 47C for seven days are summarized
in Tab. 3. It has been suggested that the starch fraction
responsible for retrogradation, as measured by DSC, is
amylopectin [19]. Therefore, the retrogradation enthalpies
(DH
r
) observed during the melting of retrograded samples
represent the relative degrees of amylopectin retro-
gradation. Data in Tab. 3 shows that oxidized starches
tended to have higher DH
r
than the native starch, indicat-
ing that oxidized starches had a higher tendency for
retrogradation. The influences of hypochlorite oxidation
on retrogradation properties of starch reported previously
are somewhat different. Lawal et al. [20] and Sandhu et al.
[21] observed a decrease in DH
r
of oxidized starches
while Kuakpetoon and Wang [5] found a slight increase.
In general, the introduction of negatively charged carboxyl
groups into the starch molecules would be expected to
hinder the chain re-association and minimize retro-
gradation. However, the results observed in our current
study indicated that the introduced carboxyl groups could
not prevent amylopectin retrogradation. It is possible that
most carboxyl groups were formed close to the branching
points of the amylopectin chains, as also suggested by
Kuakpetoon and Wang [5], or on the amylose molecules.
In such positions, they were not very effective in prevent-
ing the recrystallization of the amylopectin chains. The
increased DH
r
observed in the oxidized starches might be
explained by another mechanism of oxidation on starch
molecules. It has been known that, in addition to forma-
tion of functional groups, oxidation also causes depolym-
erization of the starch molecules. An increase in the DH
r
of
oxidized starch might be due to the degradation of long-
chain amylopectin or even amylose molecules, produc-
ing dextrins with an appropriate length for retro-
© 2009 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.starch-journal.com
98 K. Sangseethong et al. Starch/Stärke 61 (2009) 92–100
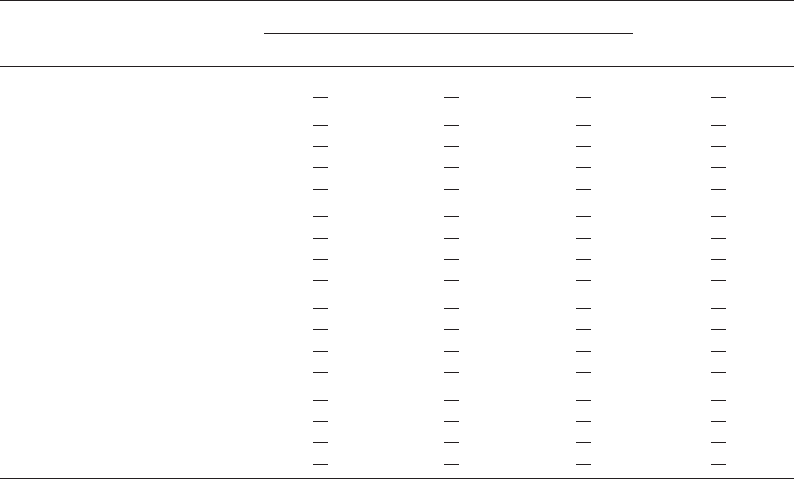
Tab. 3. Retrogradation properties of native and oxidized cassava starches.
Reaction pH Reaction
time [min]
Transition temperature [7C] Retrogradation
enthalpy [J/g]
T
o
T
p
T
c
Native - 42.80 1 0.25 53.85 1 0.35 61.98 1 0.12 6.13 1 0.21
8 30 42.35
1 0.11 54.65 1 0.07 65.09 1 0.16 7.08 1 0.02
60 42.67
1 0.13 54.60 1 0.14 64.82 1 0.03 7.06 1 0.12
120 43.09
1 0.20 54.60 1 0.28 64.89 1 0.35 7.79 1 0.24
300 43.37
1 0.13 54.85 1 0.07 64.87 1 0.29 8.47 1 0.20
9 30 40.10
1 0.25 51.90 1 0.14 63.67 1 0.05 6.61 1 0.31
60 40.66
1 0.07 52.30 1 0.00 63.76 1 0.01 6.33 1 0.05
120 41.25
1 0.40 52.85 1 0.21 63.85 1 0.01 6.27 1 0.03
300 41.46
1 0.10 52.90 1 0.00 63.70 1 0.03 6.18 1 0.15
10 30 42.18
1 0.00 53.00 1 0.00 62.86 1 0.07 5.95 1 0.02
60 42.69
1 0.21 52.75 1 0.07 62.73 1 0.02 6.29 1 0.16
120 42.58
1 0.23 53.05 1 0.21 62.64 1 0.13 6.81 1 0.23
300 42.93
1 2.60 53.85 1 1.73 63.94 1 1.32 6.68 1 1.06
11 30 39.80
10.22 51.50 1 0.28 62.57 1 0.21 6.44 1 0.03
60 40.47
1 0.22 52.15 1 0.21 62.93 1 0.21 6.67 1 0.09
120 41.35
1 0.05 52.80 1 0.14 63.50 1 0.47 7.00 1 0.20
300 42.08
1 0.06 53.15 1 0.07 63.82 1 0.23 7.23 1 0.07
gradation [5]. Results in Tab. 3 suggested that starch oxi-
dized under various reaction conditions might have dif-
ferent degradation patterns resulting in oxidized products
with different degrees of amylopectin retrogradation.
3.5 Light transmittance of starch paste
The tendency for retrogradation of native and oxidized
starches was also determined by following the changes in
light transmittance of starch pastes during storage at 47C
for seven days (Fig. 3). The initial transmittance of the
paste from native starch was at an intermediate level
(about 53%). As suggested by Craig et al. [22], the mod-
erate clarity and whiteness observed in the paste of native
cassava starch was attributed to the association of starch
molecules to form junction zones, which occurred shortly
after cooling the gelatinized paste. On the other hand,
pastes prepared from oxidized starches showed much
higher initial transmittance than native starch, indicating
that oxidized starch had a lower tendency for molecular
re-association. The presence of hydrophilic functional
groups, especially carboxyl groups, in oxidized starches
might be responsible for the higher transmittance, as
supported by the positive correlation between the car-
boxyl content and the initial light transmittance of oxi-
dized starches (Figs. 2 and 3). It has been suggested that
the early development of starch retrogradation is domi-
nated by the association of amylose [23]. Therefore, it is
possible that during starch oxidation most of or at least
part of the carboxyl groups were formed on amylose
molecules; thus, effectively retarding their re-association.
During cold storage, the transmittance of native starch
paste decreased rapidly (reaching 6% after one day stor-
age), indicating a progressive increase in the extent of
starch retrogradation. On the other hand, much less
changes in transmittance were observed for the oxidized
starch pastes, and the extent of changes was strongly
related to the amount of carboxyl group present in the
starch. Oxidized starches produced under the milder
alkaline conditions (pH 8 and 9), which had higher carboxyl
contents exhibited constant light transmittance through-
out the storage period whereas starches modified at the
higher alkaline levels (pH 10 and 11) containing lower car-
boxyl contents showed larger decrease in light transmit-
tance of starch pastes. It appeared that the more carboxyl
groups the starch contained, the smaller changes were
observed in the light transmittance. Unlike DSC results
which demonstrated that hypochlorite oxidation could not
prevent retrogradation of amylopectin, the results
observed from the changes in light transmittance of starch
paste indicated that the carboxyl groups introduced to
oxidized starch could effectively prevent starch retro-
gradation. The discrepancy of results between DSC and
light transmittance is possibly due to the differences in the
nature of these two analytical techniques. To analyze ret-
rogradation by DSC, a high starch concentration is
required (30% in this case). The gelatinization of starch in a
calorimeter pan produces swollen but nondisrupted
© 2009 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.starch-journal.com

Starch/Stärke 61 (2009) 92–100 Physicochemical Properties of Oxidized Cassava Starch 99
Fig. 3. Light transmittance of starch pastes from native and oxidized cassava starches prepared with different reaction pH
and times during storage at 47C for 7 days. Native starch (
), oxidized starch prepared with reaction time of 30 min ( ), 60
min (
), 120 min ( ) and 300 min ( ).
granules. The pan is then subjected to retrogradation
conditions, and the contents are analyzed by DSC [24]. In
such conditions, the localized carboxyl groups formed in
starch molecules, especially those on amylose and on the
branching points of amylopectin, might not be very
effective in preventing the re-crystallization of amylo-
pectin chains. In contrast, to analyze retrogradation ten-
dency by light transmittance, a low-concentrated starch
slurry is heated in a boiling water bath with continuous
stirring. This condition facilitates granule swelling and
promotes starch molecular dispersion. The starch mole-
cules carrying carboxyl groups are mingled with mole-
cules that do not contain functional groups. In this way,
the carboxyl groups could sterically hinder the aggrega-
tion of starch molecules, resulting in a lower degree of
retrogradation.
4 Conclusion
Hypochlorite oxidation under alkaline conditions tended
to favor the formation of carboxyl over carbonyl groups.
The alkalinity levels during the modification process play
an important role in determining the physicochemical
properties of the oxidized starch. Oxidation conducted
under milder alkaline conditions produced higher
amounts of functional groups; however, the extent of
viscosity reduction was much greater when oxidation
was conducted under condition with higher alkalinity.
The gelatinization temperature and the enthalpy of
gelatinization of oxidized starches were lower than
those of native starch. The presence of carboxyl groups
appeared to be responsible for the decrease in the
gelatinization temperature of oxidized starches. DSC
study revealed that hypochlorite oxidation could not
prevent the re-association of amylopectin chains. On
the other hand, the results from light transmittance of
starch paste during cold storage suggested that car-
boxyl groups introduced to oxidized starch could effec-
tively retard starch retrogradation.
Acknowledgement
This work was supported by the National Center for
Genetic Engineering and Biotechnology (BIOTEC), the
National Science and Technology Development Agency,
Ministry of Science and Technology, Thailand.
© 2009 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.starch-journal.com
100 K. Sangseethong et al. Starch/Stärke 61 (2009) 92–100
References
[1] R. L. Whistler, E. G. Linke, S. Kazeniac: Action of alkaline
hypochlorite on corn starch amylose and methyl 4-O-
methyl-D-glucopyranosides. J. Am. Chem. Soc. 1956, 78,
4704–4709.
[2] R. L. Whistler, R. Schweiger: Oxidation of amylopectin with
hypochlorite at different hydrogen ion concentrations. J.
Am. Chem. Soc. 1957, 79, 6460–6464.
[3] P. Forssell, A. Hamunen, K. Autio, T. Suortti, K. Poutanen:
Hypochlorite oxidation of barley and potato starch. Starch/
Stärke 1995, 47, 371–377.
[4] D. Kuakpetoon, Y. J. Wang: Characterization of different
starches oxidized by hypochlorite. Starch/Stärke 2001, 53,
211–218.
[5] D. Kuakpetoon, Y. J. Wang: Structural characteristics and
physicochemical properties of oxidized corn starches vary-
ing in amylose content. Carbohydr. Res. 2006, 341, 1896–
1915.
[6] J. Schmorak, M. Lewin: The chemical and physicochemical
properties of wheat starch mildly oxidized with alkaline
sodium hypochlorite. J. Polym. Sci.: Part A 1963, 1, 2601–
2620.
[7] J. Schmorak, D. Mejzler, M. Lewin: A kinetic study of the
mild oxidation of wheat starch by sodium hypochlorite in the
alkaline pH range. J. Polym. Sci. 1961, 49, 203–216.
[8] K. F. Patel, H. U. Mehta, H. C. Srivastava: Kinetic and
mechanism of oxidation of starch with sodium hypochlorite.
J. Appl. Polym. Sci. 1974, 18, 389–399.
[9] C. H. Hullinger, R. L. Whistler: Oxidation of amylose with
hypochlorite and hypochlorous acid. Cereal Chem. 1951,
28, 153–157.
[10] K. Sangseethong, K. Sriroth: Effect of hypochlorite levels on
the modification of cassava starch. Zywnosc Technologia
Jakosc 2002, 9, 191–197.
[11] Y. J. Wang, L. Wang: Physicochemical properties of com-
mon and waxy corn starches oxidized by different levels of
sodium hypochlorite. Carbohydr. Polym. 2003, 52, 207–217.
[12] O. B. Wurzburg: Converted starches, in Modified Starches:
Properties and Uses (Ed. O. B. Wurzburg) CRC Press, Boca
Raton, Florida, 1986.
[13] Food and Agriculture Organization (FAO): FAO Food and
Nutrition Paper 52 Addendum 9. Food and Agriculture
Organization of the United Nations, 2001.
[14] M. R. Jacobson, M. Obanni, J. N. BeMiller: Retrogradation
of starches from different botanical sources. Cereal Chem.
1997, 74, 511–518.
[15] M. W. Rutenberg, D. Solarek: Starch derivatives: production
and uses, in Starch: Chemistry and Technology,2
nd
ed. (Eds.
R. L. Whistler, J. N. BeMiller, E. F. Paschall) Academic Press,
New York, 1984.
[16] J. N. BeMiller: Alkaline degradation of starch, in Starch:
Chemistry and Technology, vol. 1 (Eds. R. L. Whistler, E. F.
Paschall) Academic Press, New York, 1965.
[17] V. V. Prey, S. Siklossy: Die Rolle der Aldehydgruppen in
hypochloritoxydierten Stärken. Stärke 1971, 23, 235–238.
[18] D. Kuakpetoon, Y. J. Wang: Locations of hypochlorite oxi-
dation in corn starches varying in amylose content. Carbo-
hydr. Res. 2008, 343, 90–100.
[19] A. A. Karim, M. H. Norziah, C. C. Seow: Methods for the
study of starch retrogradation. Food Chem. 2000, 71, 9–36.
[20] O. S. Lawal, K. O. Adebowale, B. M. Ogunsanwo, L. L.
Barba, N. S. Ilo: Oxidized and acid thinned starch deriva-
tives of hybrid maize: functional characteristics, wide-angle
X-ray diffractometry and thermal properties. Int. J. Biol.
Macromol. 2005, 35, 71–79.
[21] K. S. Sandhu, M. Kaur, N. Singh, S. T. Lim: A comparison of
native and oxidized normal and waxy corn starches: physi-
cochemical, thermal, morphological and pasting properties.
Lebensm. Wiss. Technol. 2008, 41, 1000–1010.
[22] S. A. S. Craig, C. C. Maningat, P. A. Seib, R. C. Hoseney:
Starch paste clarity. Cereal Chem. 1989, 66, 173–182.
[23] M. J. Miles, V. J. Morris, P. D. Orford, S. G. Ring: The roles of
amylose and amylopectin in the gelation and retrogradation
of starch. Carbohydr. Res. 1985, 135, 271–278.
[24] M. R. Jacobson, J. N. BeMiller: Method for determining the
rate and extent of accelerated starch retrogradation. Cereal
Chem. 1998, 75, 22–29.
(Received: July 10, 2008)
(Revised: August 8, 2008)
(Accepted: September 10, 2008)
© 2009 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.starch-journal.com
