
Clinical guide
Clinical guide
English
i
Contents
Welcome ........................................................................................................................................... 1
Indications for use ...................................................................................................................... 1
Contraindications ........................................................................................................................ 1
Adverse effects .......................................................................................................................... 1
Software functionality and device data ............................................................................................ 2
At a glance ........................................................................................................................................ 3
About your device ...................................................................................................................... 4
Therapy Information ......................................................................................................................... 5
AutoSet mode ............................................................................................................................ 5
Normal airway ...................................................................................................................... 5
Flow limitation...................................................................................................................... 5
Snore .................................................................................................................................... 6
Apnea ................................................................................................................................... 6
AutoSet for Her mode ................................................................................................................ 7
CPAP mode ................................................................................................................................ 7
Reporting .................................................................................................................................... 7
Central sleep apnea detection ............................................................................................. 7
Cheyne-Stokes respiration detection .................................................................................. 8
Respiratory effort related arousals reporting ...................................................................... 9
Comfort features ............................................................................................................................... 9
Ramp........................................................................................................................................... 9
Expiratory Pressure Relief ................................................................................................... 9
AutoSet Response ............................................................................................................. 10
About the heated tubing .......................................................................................................... 10
Climate Control .................................................................................................................. 10
Setting up your device .................................................................................................................... 12
Navigating the touch screen ........................................................................................................... 14
Accessing the Clinical menu .................................................................................................... 15
Adjusting Clinical settings ........................................................................................................ 17
Connecting your AirSense 11 device and smart device ......................................................... 17
Settings Menu ................................................................................................................................. 18
Therapy Settings ...................................................................................................................... 18
Comfort Settings ...................................................................................................................... 18
Options ..................................................................................................................................... 19
Configuration ............................................................................................................................ 19
Setting the time zone ............................................................................................................... 20
Restoring settings and erasing data ........................................................................................ 20
Starting/stopping therapy ............................................................................................................... 21
Viewing sleep data and option controls ......................................................................................... 22
Supplemental oxygen ..................................................................................................................... 23
Cleaning and caring for the device ................................................................................................. 24
Disassembling .......................................................................................................................... 25
Cleaning .................................................................................................................................... 25
Checking ................................................................................................................................... 26
Replacing the air filter .............................................................................................................. 26
Reassembling .................................................................................................................................. 26
Preparing the device for use between patients.............................................................................. 27
Disassembling .......................................................................................................................... 28
Device enclosure ...................................................................................................................... 29
Air tubing, outlet connector and HumidAir 11 tub .................................................................. 29
ii
Disinfection .............................................................................................................................. 30
Reassembling ........................................................................................................................... 30
Packing and storing .................................................................................................................. 31
Data management and therapy compliance .................................................................................. 32
Remote monitoring .................................................................................................................. 32
Data storage .................................................................................................................................... 33
Troubleshooting ............................................................................................................................. 34
General Warnings ........................................................................................................................... 36
Technical specifications ................................................................................................................. 37
Symbols .......................................................................................................................................... 41
Limited warranty ............................................................................................................................ 42
Further information ........................................................................................................................ 43

English 1
Welcome
The AirSense 11 AutoSet™ (including AutoSet for Her) device is ResMed's premium auto-adjusting
pressure device. The AirSense 11 Elite and the AirSense 11 CPAP are ResMed's Continuous Positive
Airway Pressure (CPAP) devices.
WARNING
Read this entire guide before using the device.
CAUTION
In the US, Federal law restricts this device to sale by or on the order of a physician.
Indications for use
AirSense 11 AutoSet (including AutoSet for Her)
The AirSense 11 self-adjusting system is indicated for the treatment of obstructive sleep apnea (OSA) in
patients weighing more than 66 lb (30 kg), including female patients with mild to moderate OSA in AutoSet
for Her mode. The AirSense 11 self-adjusting system is intended for home and hospital use.
AirSense 11 CPAP (including Elite)
The AirSense 11 CPAP system is indicated for the treatment of obstructive sleep apnea (OSA) in patients
weighing more than 66 lb (30 kg). The AirSense 11 CPAP system is intended for home and hospital use.
Contraindications
Positive airway pressure therapy may be contraindicated in some patients with the following pre-existing
conditions:
• severe bullous lung disease
• pneumothorax or pneumomediastinum
• pathologically low blood pressure, particularly if associated with intravascular volume depletion
• dehydration
• cerebrospinal fluid leak, recent cranial surgery, or trauma.
Adverse effects
Patients should report unusual chest pain, severe headache, or increased breathlessness to their
prescribing physician. An acute upper respiratory tract infection may require temporary discontinuation of
treatment.
The following side effects may arise during the course of therapy with the device:
• drying of the nose, mouth, or throat
• nosebleed
• bloating
• ear or sinus discomfort
• eye irritation
• skin rashes.
2
Software functionality and device data
This ResMed device is a smart device and includes software functionalities which allow it to be connected
to the cloud so that users and their care providers can access data about therapy remotely, receive regular
upgrades to the device and much more. Check out https://myair.resmed.com/ to learn about ResMed’s
patient coaching application, myAir™.
Software License
License Grant. Subject to the terms and conditions below, ResMed grants you, the owner and/or user of
this device, a perpetual, non-exclusive, non-sublicensable, personal, limited license to use the ResMed
Software solely in connection with the use of this device. All other rights are reserved by ResMed. You
will be deemed to have transferred and assigned this license to any person that acquires the owner’s or
the user’s rights in this device.
License Restrictions. Software included on or with this device is owned by or licensed to ResMed (the
"ResMed Software"). Neither the ResMed Software nor any intellectual property rights in the ResMed
Software are sold or assigned by ResMed. No person or entity is licensed or authorized to (a) reproduce,
distribute, create derivative works, modify, display, perform, decompile or attempt to discover the source
code for the ResMed Software, (b) remove or attempt to remove the ResMed Software from the ResMed
product, or (c) reverse engineer or disassemble the ResMed product or the ResMed Software. For
avoidance of doubt, the foregoing restrictions are not intended to limit any licensee’s rights to software
code incorporated into or distributed with the ResMed Software and licensed under the terms of any open
source, free or community software license (collectively, "Open Source Software").
Over-the-Air Download of Software Updates. If the device is connected to the cloud, then the ResMed
Software on the device will automatically and periodically download updates and upgrades to the ResMed
Software on the device. Such downloads may be done by various means including, but not limited to,
using Bluetooth®
wireless technology, WiFi and/or cellular networks and combinations of various wireless
technologies and services. Such updates to the ResMed Software might include, without limitation, bug
fixes, error corrections, security patches, and new versions and releases of the ResMed Software that may
include changes to existing features or functions and/or the addition of new features and functions.
Use of Device Data
When you use this device it gathers and records data about your use and, if your device connectivity is
enabled, the device sends certain data to ResMed via the cloud to enable ResMed to deliver various
benefits to you and your care provider(s). Additionally, some of that data may be used by ResMed (1) to
comply with its legal obligations; these legal obligations include collection and analysis of device data for
medical device post market surveillance and vigilance, and compliance with these legal obligations includes
assessing if ResMed is required to implement actions to improve device safety, usability and performance,
and (2) to perform health-related research, study and/or evaluation for specific scientific and medico-
economic purposes. ResMed will only use your device data in compliance with applicable laws and
regulations in your country or region (for example the GDPR (Regulation (EU) 2016/679 of the European
Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the
processing of personal data and on the free movement of such data), the MDR (Regulation (EU) 2017/745
of the European Parliament and of the Council of 5 April 2017 on Medical Devices)) in the European Union,
and, as applicable, HIPAA (the Health Insurance Portability and Accountability Act of 1996) in the USA).
Depending on the data protection or privacy laws of your country or region your device data may constitute
your personal data. If so, ResMed has the obligation to inform you about your rights and freedoms for our
use of your personal data. You can find more details related to our use of your data, your rights to access,
rectify, erase, restrict or object at https://www.resmed.com/myprivacy/.

English 3
At a glance
WARNING
Use only recommended ResMed masks and accessories or other vented masks as recommended by
the prescribing doctor with this device. Using these components allows normal breathing and
prevents potential asphyxiation.
The AirSense 11 system includes the following:
• Device
• HumidAir™11 Standard tub
• HumidAir 11 Cleanable tub
• ClimateLineAir™11 heated tubing or SlimLine™ tubing
• 65W AC adaptor
• Travel bag
• SD card (not available in all devices).
A range of accessories is available for use with this device including:
• Air tubing (ClimateLineAir 11, SlimLine and Standard)
• HumidAir 11 Standard tub (Single patient re-use - cannot be reprocessed)
• HumidAir 11 Cleanable tub (Multi patient re-use - can be reprocessed)
• Endcap which allows use without the humidifier
• Air11™ Filter - standard
• Air11 Filter - hypoallergenic
• DC-DC Converter
• SD card
• SD card cover
Notes:
• The AirSense 11 device is compatible with ResMed masks. For a complete list, see the Mask/Device
compatibility list on ResMed.com/downloads/devices.
• It is the responsibility of the equipment provider or clinician to ensure that parts and accessories are
compatible with this device prior to use by the patient.
• The HumidAir 11 Standard tub and the HumidAir 11 Cleanable tub are the only water tubs used with
the AirSense 11 device.
• The ClimateLineAir 11 is the only heated tubing that is compatible with the AirSense 11 device.
• Ensure the patient has an approved ResMed power supply for the region they are using the device.

4
About your device
Description
Purpose
1
Start Therapy/ Standby button
Press to start/stop therapy.
The LED indicator is green during standby mode, and white during
therapy, Test Drive, and Mask Fit functions.
2
Display touch screen
Navigates between functions and displays information on the
operating status of the device.
3
HumidAir 11 tub
Water tub that provides heated humidification.
4
Device label
Contains information relevant to the device.
5
Outlet connector
Connects the air tubing
6
Power inlet
Connects the power cord
7
Air inlet filter cover
Contains the air filter
8
SD card cover
Removable cover that protects the SD card slot.
The LED indicator is blue when data is written to the SD card.
9
SlimLine tubing
Non-heated air tubing
10
ClimateLineAir 11 tubing
Heated air tubing
Notes:
• If the Start therapy/ Standby button has a flashing white light, a system error has occurred. Refer to the
Troubleshooting section for more information.
• Use this device only as directed by your physician or healthcare provider.

English 5
Therapy Information
The following modes are available on the AirSense 11 device:
Device
Modes available
AutoSet
AutoSet for Her
CPAP
AirSense 11 AutoSet
AirSense 11 CPAP
AirSense 11 Elite
AutoSet mode
The treatment pressure required by the patient may vary due to changes in sleep state, body position and
airway resistance. In AutoSet mode, the device provides only that amount of pressure required to maintain
upper airway patency.
The device analyzes the state of the patient’s upper airway on a breath-by-breath basis and delivers
pressure within the allowed range according to the degree of obstruction. The AutoSet algorithm adjusts
treatment pressure as a function of three parameters: inspiratory flow limitation, snore, and apnea.
Normal airway
When the patient is breathing normally, the inspiratory flow measured by the device as a function of time
shows a typically rounded curve for each breath.
Flow limitation
As the upper airway begins to collapse, the shape of the inspiratory flow-time curve changes. The
AirSense 11 recognizes and treats traditional as well as less common flow-limited breath wave forms.

6
Snore
Snoring is sound generated by vibrations of the walls of the upper airway. It is often preceded by flow
limitation or a partial obstruction of the airway.
Apnea
The enhanced AutoSet algorithm detects both obstructive and central apneas. If an apnea occurs, the
device responds appropriately.
Obstructive apnea
An obstructive apnea is when the upper airway becomes severely limited or completely obstructed.
AutoSet generally prevents obstructive apneas from occurring by responding to flow limitation and snoring.
If an obstructive apnea occurs, the device will respond by increasing pressure.
Central apnea
During a central apnea, the airway will remain open, but there is no flow. When a central apnea is
detected, the device responds appropriately by not increasing pressure.

English 7
AutoSet for Her mode
AutoSet for Her mode is based on key aspects of ResMed’s AutoSet algorithm and delivers therapeutic
responses tailored to the characteristics of female OSA patients.
The AutoSet for Her is similar to ResMed’s AutoSet algorithm with the following modifications:
• Reduced rate of pressure increments designed to help prevent arousals.
• Slower pressure decays.
• Treats apneas up to 12 cm H
2
O (12 hPa) and continues to respond to flow limitation and snore up to
20 cm H
2
O (20 hPa).
• Minimum pressure (Min. Pressure) that adjusts according to the frequency of apneas:
If two apneas occur within a minute, the pressure reached in response to the second apnea will
become the new minimum treatment pressure until the next treatment session.
Patients who use AutoSet for Her will still get the benefits of ResMed's AutoSet technology including
improved sensitivity to flow-limitation and Central Sleep Apnoea Detection with Forced Oscillation
Technique.
CPAP mode
In CPAP mode, a fixed pressure is delivered—with optional Expiratory Pressure Relief (EPR
™
).
Reporting
The AirSense 11 reports Respiratory Effort Related Arousals (RERA), and detects Central Sleep Apnea
(CSA) and Cheyne-Stokes Respiration (CSR). The summary and detailed data of these parameters are
available to view on ResMed's patient compliance software (data availability depends on device mode and
parameter measured).
Central sleep apnea detection
Available in all modes on the AirSense 11 AutoSet and the AirSense 11 Elite.
The device detects both obstructive and central sleep apneas (CSA). CSA detection uses the Forced
Oscillation Technique (FOT) to determine the state of the patient’s airway during an apnea. When an apnea
has been detected, small oscillations in pressure [1 cm H
2
O (1 hPa) peak-to-peak at 4 Hz] are added to the
current device pressure. The CSA detection algorithm uses the resulting flow and pressure (determined at
the mask) to measure the airway patency.

8
Cheyne-Stokes respiration detection
Available in all modes on the AirSense 11 AutoSet and the AirSense 11 Elite.
Cheyne-Stokes respiration (CSR) is a form of sleep-disordered breathing characterized by a periodic waxing
and waning of respiration. The waxing periods (hyperpneas, typically 40 seconds in length) can include
large gasping breaths that tend to arouse the patient while the waning periods (hypopneas or apneas,
typically 20 seconds in length) cause blood oxygen desaturations.
The following example shows a typical CSR period.
The following example suggests periodic breathing due to the frequently occurring apneas. However,
when looking closely at the shape of the hyperpneas it can be seen that it is a typical OSA period.
The AirSense 11 device reports the time during therapy in which it detected breathing patterns indicative
of CSR. It analyzes the patient's respiratory flow for apnea/hypopnea events, calculates the time between
these events, and characterizes the shape of breathing between them.

English 9
Respiratory effort related arousals reporting
Respiratory Effort Related Arousals (RERA) reporting is available on the AirSense 11 AutoSet and AirSense
11 Elite in all modes.
RERAs are periods of increasing respiratory effort which are terminated by an arousal. Increasing
respiratory effort will be seen as airflow limitation.These flow-based RERA events are logged and stored as
summary and/or detailed data and can then be viewed in one of ResMed's patient management systems.
Comfort features
Ramp
Designed to make the beginning of treatment more comfortable, ramp is available in all modes.
In AutoSet and AutoSet for Her mode, ramp time defines the period during which the pressure gradually
increases from a lower more comfortable start pressure to the minimum treatment pressure before the
auto-adjusting algorithm commences.
In CPAP mode, the pressure increases from a low pressure (Start Pressure) to the prescribed treatment
pressure.
Ramp Time can be set to Off, 5 to 45 minutes or Auto. When Ramp Time is set to Auto, the device will
detect sleep onset and then gradually increase from the start pressure to the minimum treatment pressure
at a rate of 1 cm H
2
O (1 hPa) per minute. However, if sleep onset is not detected, the device will reach the
target pressure within 30 minutes.
Expiratory Pressure Relief
Designed to make therapy more comfortable, Expiratory Pressure Relief (EPR) maintains optimal treatment
for the patient during inhalation and reduces the delivered mask pressure during exhalation.
EPR On—EPR is enabled.
Off—EPR is disabled.
The following settings are only available if EPR is On:
EPR Type
Full Time—If set to Full Time, EPR is enabled for the whole therapy session.
Ramp Only—If set to Ramp Only, EPR is only enabled during ramp time.
EPR Level
1, 2, 3 cm H
2
O (1, 2, 3 hPa)
When EPR is enabled, the delivered pressure will not drop below a minimum pressure of 4 cm H
2
O
(4 hPa), regardless of the settings.
10
AutoSet Response
AutoSet mode (AirSense 11 AutoSet device only).
For patients who are sensitive to faster changes in pressure during therapy, AutoSet Response can be set
to either Standard or Soft. If set to soft, patients will receive gentler pressure rises during therapy.
Patients who use the AutoSet Response feature will still get the benefits of ResMed's AutoSet technology
including improved sensitivity to flow-limitation and CSA Detection with Forced Oscillation Technique.
About the heated tubing
The ClimateLineAir 11 is a heated breathing tube that delivers air to a compatible mask. When used with
the device humidifier tub, ClimateLineAir 11 heated air tubing allows you to use the Climate Control
feature.
Note: Not all types of air tubing are available in all regions.
Climate Control
Climate Control is an intelligent system that controls the humidifier and the ClimateLineAir heated air
tubing. This feature:
• delivers comfortable humidity level and temperature during therapy
• maintains the set temperature and relative humidity during sleep to prevent dryness in the nose and
mouth
• can be set to either Auto or Manual
• is only available when both the ClimateLineAir 11 and HumidAir 11 tub are attached.
Climate Control - Auto setting
Auto is the recommended and default setting. It is designed to make therapy as easy as possible so there
is no need to change the temperature or humidity settings.
• Sets the tube temperature to Auto (80ºF/27ºC). If the air in the mask is too warm or too cold, you can
adjust the tube temperature to anywhere from 60 to 86ºF (16 to 30ºC) or turn it off completely
• Adjusts the humidifier output to maintain a constant, comfortable humidity level of 85% relative
humidity
• Protects against rainout (water droplets in the heated air tubing and mask).
Climate Control - Manual setting
Manual is designed to offer more flexibility and control over settings and offers the following:
• Temperature and humidity can be adjusted to find the most comfortable setting
• Temperature and humidity level can be set independently
• Rainout protection is not guaranteed. If rainout does occur, first try increasing the tube temperature
• If the air temperature becomes too warm and rainout continues, try decreasing the humidity.
Notes:
• If Climate Control is set to Manual, the Auto Tube Temperature setting is not available.
• The temperature and humidity settings are not measured values.
Tube Temperature
The temperature sensor located at the mask end of the ClimateLineAir 11 heated air tubing enables the
system to automatically control the temperature of the air delivered to the patient. This ensures the
temperature of the air delivered to the patient does not fall below the set minimum temperature, therefore
maximizing breathing comfort for the patient.
Humidity Level
The humidifier moistens the air and is designed to make therapy more comfortable.
• If the patient is getting a dry nose or mouth, turn up the humidity
• If the patient is getting any moisture in the mask turn down the humidity.
English 11
• The Humidity Level can be set to: Off or between 1 and 8, where 1 is the lowest humidity setting and
8 is the highest humidity setting.
For each humidifier setting, the Climate Control system delivers a constant amount of water vapor, or
absolute humidity (AH), to the patient's upper airway.
Automatic Adjustment
The humidifier and ClimateLineAir 11 heated air tubing are controlled by the Climate Control algorithm to
deliver constant humidity and temperature outputs. The system adjusts automatically to changes in:
• ambient room temperature and humidity values
• flow due to pressure changes
• flow due to mask or mouth leak.

12
Setting up your device
WARNING
Do not use any additives in the humidifier tub (eg, scented oils or perfumes). These may reduce
humidification output and/or cause deterioration of the tub materials.
CAUTION
Use only ResMed parts (eg, air inlet filter, power supplies), masks and accessories with the machine.
Non ResMed parts may reduce the effectiveness of the treatment, may result in excess carbon
dioxide rebreathing and/or damage the machine. For compatibility information, refer to
ResMed.com for more information.
When using the humidifier tub:
• Always place the device on a level surface, lower than your head, to prevent the mask and air
tubing from filling with water.
• Do not overfill the humidifier tub as water may enter the device and air tubing.
• Do not fill the humidifier tub with hot water as this could lead to excessive air temperature at the
mask. Ensure the water is cooled to room temperature before filling the humidifier tub.
• Do not place the device on its side while the humidifier is attached as water might get into the
device and reduce motor life.
When setting up the AirSense 11 system:
• Do not place the power supply where it can be bumped, stepped on, or where someone is likely
to trip over the power cord
• Do not block the air tubing and/or air inlet of the device while in operation as this could lead to
overheating of the device
• Keep the area around the device dry, clean and clear of anything (eg, clothes or bedding) that
could block the air inlet or cover the power supply unit
• Ensure the system is correctly set up. Incorrect system setup may result in incorrect mask
pressure reading.
When using a mask:
• Use only vented masks recommended by ResMed or by the prescribing doctor with this device
• Fitting the mask without the device blowing air can result in rebreathing of exhaled air
• Make sure that the mask vent holes are kept clear and unblocked to maintain the flow of fresh air
into the mask.

English 13
To set up the device:
1. Place the device on a stable level surface.
2. Connect the power cord into the power inlet at the rear of the device. Connect one end of the power
cord into the AC adaptor and the other end into the power outlet. Ensure the device is set up and
connected to power to enable settings to be applied wirelessly to the device if required.
3. Connect the air tubing firmly to the outlet connector at the rear of the device.
4. Open the humidifier tub and fill it with distilled water up to the maximum water level mark. The
humidifier tub must be removed from the device before adding water. The humidifier tub has a
maximum capacity of 380 mL.
5. Close the humidifier tub and insert it into the side of the device.
6. Connect the free end of the air tubing firmly onto the assembled mask.
See the mask user guide for detailed information.
Recommended masks for use with this device are listed on ResMed.com.
Notes:
• Do not insert any USB cable into the AirSense 11 device or attempt to plug the AC adaptor into a USB
device. This may cause damage to the AirSense 11 device or USB device.
• The electrical connector end of the heated air tubing is only compatible with the air outlet at the device
end and should not be fitted to the mask.
• Do not use electrically conductive or anti-static air tubing.
14
Navigating the touch screen
The AirSense 11 device operates via a display touch screen. This allows you to access, view and change
therapy and device settings and to track the sleep health progress of your patient.
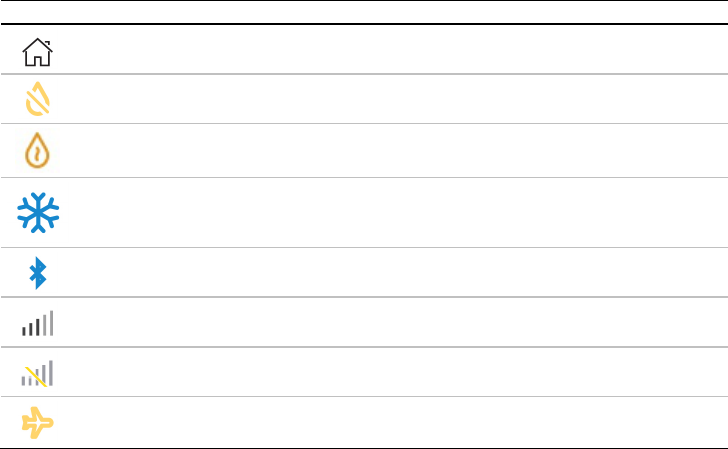
The status bar at the top of the screen may display icons at different times and may include:
Icon
Description
Purpose
Home Screen
Return to the Clinical Home screen at any time.
Humidifier fault
Detects fault in the humidifier. Therapy will run without heating.
Humidifier warming
Water in the humidifier tub is pre-heating.
Humidifier cooling
Water in the humidifier tub is cooling.
Bluetooth connected
Device is successfully connected via Bluetooth wireless
technology.
Cellular signal strength
Indicates the strength of cellular connectivity.
No cellular connection
Cellular coverage is not available.
Airplane mode
Device is in airplane mode.

English 15
Accessing the Clinical menu
From the Welcome screen, tap CLINICIAN
The
Clinician
handover screen will appear.
Tap OK to continue or BACK to return to previous screen.
The Home screen will appear. This is also the Patient Home screen.
The menu options are:
MY OPTIONS: for the patient to view and adjust therapy settings (eg,
Adjust Ramp time)
MY SLEEP VIEW: for the patient to track their sleep health (eg, check
the number of hours used last night or mask status)
MORE: Additional features such as Mask Fit or switch to Airplane
Mode.
To access Clinical mode:
From the Home screen, press two fingers anywhere on the screen
for 3 seconds to access the Clinical Home screen.
From this screen, you can access:
SETTINGS: Set up or adjust therapy settings for the patient.
SLEEP VIEW: Track the patients sleep health
EXIT: Return to the Home screen (Patient View)
Notes:
• Patient screens have a black background. Clinical screens have a white background.
• Menu options will also vary by treatment mode. Refer to the Settings menu to view the settings for
each therapy mode.

16
Using the touch screen:
There are two actions to navigate through the touch screen:
Swipe:
Swipe up or down the screen to view menu options..
Tap: Select a parameter setting to update. For other parameters
(eg Pressure Relief, Airplane mode), tap the parameter to turn it on
or tap to turn it off .
To update settings:
Tap SETTINGS. The Settings list will display.
1. Tap the preferred setting (eg, Response)
2. Tap on the desired value.
3. Tap OK to confirm the change or CANCEL to go back to the
previous screen.
To exit the Clinical menu:
1. Tap
at any time to return to the Clinical Home screen.
2. Tap EXIT to leave the Clinical menu.
English 17
Adjusting Clinical settings
The AirSense 11 settings and equipment (including accessories) must be configured for each patient. The
settings and equipment used should be periodically reassessed to ensure optimal therapy.
All parameters relating to a patient's therapy and device configuration are managed through the Settings
menu.
Connecting your AirSense 11 device and smart device
myAir™ is a smartphone app that guides the patient through the setup process. This includes device setup
videos, mask fitting videos, trying therapy using the Test Drive feature, and tracking their sleep health
progress.
The app is not required to operate the AirSense 11 device.
Before pairing the AirSense 11 device to a smartphone, ensure the app's latest version is installed on the
smartphone. If not, download the app from the App Store
®
or on Google Play
®.
Pair the AirSense 11 device
to your phone. To set up the app, go to the MORE menu.
1. Ensure the AirSense 11 device is set up correctly and plugged into a power source.
2. Launch the myAir app. Tap Continue.
3. Follow the prompts on the myAir app to complete the Bluetooth connection. AirSense 11 is now
connected to the app. The Bluetooth connection symbol appears on the status bar to confirm the
connection between the AirSense 11 device and the smartphone.
4. Tap Done.

18
Settings Menu
Therapy Settings
Parameter Description
Mode
Range
AutoSet
AutoSet
for Her
CPAP
Mode Sets the therapy mode available on
the device.
Pressure Range Sets the pressure range for
treatment.
Select Min Pres.:
4-20 cm H
2
O (hPa), 0.2cm H
2
O (hPa)
increments.
Select Max Pres.:
4-20 cm H
2
O (min-20 hPa) 0.2 cm
H
2
O (0.2 hPa) increments.
Set Pressure Sets the fixed treatment pressure.
4-20 cm H
2
O (4-20 hPa), 0.2 cm H
2
O
(0.2 hPa) increments.
Comfort Settings
Parameter Description
Mode
Range
AutoSet
AutoSet
for Her
CPAP
AutoSet
response
Set to Standard or Soft.
If Soft is selected, patients will
receive gentler pressure rises
during therapy.
Standard/Soft
Ramp Time If Auto is selected, the device will
detect sleep onset and
automatically rise to the prescribed
treatment pressure.
Off/ 5-45 Mins / Auto
Start Pressure Set the pressure at the start of
ramp, up to treatment pressure.
4-Set pressure level,
0.2 cm H
2
O (0.2 hPa) increments
EPR Enable/ disable EPR.
On/ Off
EPR Type Available when EPR is enabled
Full time/ Ramp Only
EPR Level Set the EPR value.
1 / 2 / 3 cm H
2
O (1 / 2 / 3 hPa)
Climate Control Available when the HumidAir 11
tub is used and ClimateLineAir 11
heated air tubing is connected.
Manual/Auto
Tube Temp Set the minimum temperature of
air delivered by heated air tubing
such as ClimateLineAir 11 .
Off/60-86°F (16-30°C), increments
of 2ºF (1°C)
Humidity Level Set the humidity level.
Off / 1-8

English 19
Options
Parameter Description Range
Patient View Set the level of access available to the patient. Simple/ Advanced
SmartStart™* Enable/disable the SmartStart feature. If you enable the SmartStart feature, the
device will start automatically when the patient breathes into the mask.
On/ Off
SmartStop* Enable/disable the SmartStop feature. If you enable the SmartStop feature, the
device will stop automatically when the patient removes the mask.
On/ Off
Care Check-In* Enable Care Check-in. A series of simple questions presented to the patient to
gain insight on their progress with sleep therapy and enables them to enroll in
the myAir application.
On/ Off
Reminders
Mask Set a recurring reminder to the patient to replace the mask. Off/ 1/ 3/ 6/ 9/ Yearly
Tube Set a recurring reminder to the patient to replace the air tubing Off/ 1/ 3/ 6/ 9/ Yearly
Filter Set a recurring reminder to the patient to replace the air filter Off/ 1/ 3/ 6/ 9/ Yearly
Humidifier Set a recurring reminder to the patient to replace the water tub Off/ 1/ 3/ 6/ 9/ Yearly
*Settings are enabled via a Tap on or tap off .
Configuration
Parameter Description Range
Language Set the display language English
Temperature
Units
Set the temperature units °F / °C
Restore
Defaults
Reset to default settings OK to restore defaults. Cancel
to return to previous menu.
Erase data Erase all data stored on the device and SD card. OK to erase data
Cancel to return to previous
menu.
About View SN, CG details, Run hours and modem provider
TimeZone Set up the correct time zone for the patient GMT time zone
Note: Not all functions are available in all regions. Functions vary based on therapy mode.
20
Setting the time zone
Before the patient is set up, ensure the correct time zone has been set. The time zone cannot be changed
once patient data has been stored on the device. The patient data will need to be erased before changing
the time zone.
The AirSense 11 device is set up with GMT (Greenwich Mean Time) time zone settings.
To change Time Zone:
1. From the Clinical Home screen, tap SETTINGS
2. Move down the menu to find CONFIGURATION options
3. Tap Time Zone
4. Select the relevant GMT setting and tap OK.
Restoring settings and erasing data
When using the device in a multi-patient environment, the device settings should be reset between patient
use.
To restore default settings:
1. From the Clinical Home screen, tap SETTINGS
2. Move down the menu to find CONFIGURATION options
3. Tap Restore Defaults
4. Tap OK to confirm or CANCEL to return to the previous screen.
To erase data from the device:
1. From the Clinical Home screen, tap SETTINGS
2. Move down the menu to find CONFIGURATION options
3. Tap Erase data
4. Tap OK to confirm or CANCEL to return to the previous screen.

English 21
Starting/stopping therapy
WARNING
The machine is not intended to be operated by persons (including children) with reduced physical,
sensory or mental capabilities without adequate supervision by a person responsible for the
patient’s safety.
To start therapy:
1.
Direct the patient to fit their mask.
2. Direct the patient to press the Start therapy/ Standby button or if the
SmartStart feature is enabled, direct them to breathe into their mask.
Therapy will begin and the Treatment screen is displayed. A dynamic
pulse wave will appear during therapy
Notes:
• The screen will fade and then go black automatically after a short period of time. Tap the screen to turn
it back on.
• If power is interrupted during therapy, the device will automatically restart therapy when power is
restored.
• The device has a light sensor that adjusts the screen brightness based on the light in the room.
To stop therapy
1. Direct the patient to remove the mask.
2. Direct the patient to press the Start therapy/ Standby button or if SmartStop is enabled, therapy will
stop automatically after a few seconds.

22
Viewing sleep data and option controls
The Sleep View screen shows sleep quality and mask seal status for the most recent therapy session. The
parameters displayed will depend on the therapy mode.
In the Patient view, there are two types of access levels: Simple and Advanced.
The Simple view is designed to:
• Make the device interaction and menu navigation easier for patients
• Provide access to the most important comfort features such as Ramp Time, Mask Fit, Humidity level
and Warmup (if humidifier is available)
The Advanced view provides highly engaged patients access to additional features to monitor their sleep
health. These include:
• EPR (if available)
• SmartStart and/or SmartStop
The Advanced view can be enabled via the Settings screen. For more information on the Patient view, see
the User Guide.
Sleep View Parameters:
Parameter Description
Used Hrs Number of hours the device has been used since last session
Pressure Average Pressure during the selected period (95th percentile for each day, average of
the 95th percentile values for periods >1 day)
Leak Average of the 95th percentile values of leak during the selected period.
AHI Apnea-Hypopnea Index - average AHI during the selected period.
Total AI Apnea Index - average total AI during the selected period
Central AI Central Apnea Index - average CAI of the Days Used in the selected period.

English 23
Supplemental oxygen
Before adding oxygen, familiarize yourself and your patient with the following warnings relating to the use
of supplemental oxygen.
WARNING
• Supplemental oxygen must not be used while smoking or in the presence of an open flame.
• When using the device with an oxygen supply, check the following:
• Starting therapy – ensure the device is on and blowing air before the oxygen supply is turned
on.
• Stopping therapy – ensure the oxygen supply is turned off first, then the device.
This will ensure oxygen does not accumulate within the device and create a risk of fire.
The device is designed to be compatible with up to 15 L/min of supplemental oxygen in all modes.
At a fixed rate of supplemental oxygen flow, the inhaled oxygen concentration will vary depending on the
pressure settings, patient breathing pattern, mask selection and the leak rate.
An oxygen connector port is required to connect supplemental oxygen to the device. Oxygen
concentration should be measured at the point of delivery to the patient.
Notes:
• Adding oxygen may affect the delivered pressure and the accuracy of the displayed and reported values
• Oxygen concentration can be affected by a partial obstruction downstream of the AirSense 11 system.
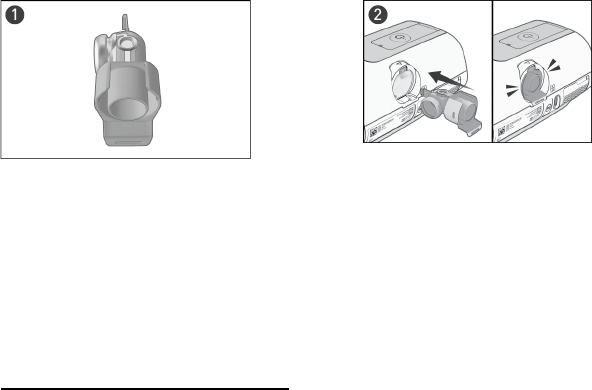
Fitting an oxygen port
1. Firmly connect the Oxygen Connector Port (A)
directly to the air outlet (B) of the flow
generator.
2. Connect the non-heated air tubing to the end
of the Oxygen Connector Port (C) as shown.
Ensure that the non-heated tube is connected
up to the indicated line (D).
3. Connect the oxygen supply tubing to the
oxygen inlet port (E) as shown.

24
Cleaning and caring for the device
WARNING
• Beware of electrocution:
• Do not immerse the device, AC Adaptor or power cord in water.
• Do not connect to power while the device is wet. Make sure that all parts are dry before
plugging it in.
• If liquids are spilled into or onto the device, unplug the device and let the parts dry.
• Always unplug the device before cleaning and ensure that all parts are dry before plugging it
back in.
• Do not perform any maintenance tasks (eg, cleaning, changing the air filter) while the device is in
operation.
• Clean the device and its components according to the schedules shown in this guide, to maintain
the quality of the device and to prevent the growth of germs that can adversely affect your
health.
• Regularly inspect power cords, cables, and power supply for damage or signs of wear.
Discontinue use and replace if damaged.
• Do not open or modify the device. There are no user serviceable parts inside. Repairs and
servicing should only be performed by an authorized ResMed service agent.
CAUTION
• Do not use bleach, chlorine, or aromatic-based solutions, moisturizing or antibacterial soaps or
scented oils to clean the device, the humidifier tub or air tubing. These solutions may cause
damage or affect the humidifier performance and reduce the life of the products. Exposure to
smoke, including cigarette, cigar or pipe smoke, as well as ozone or other gases, may damage
the device. Damage caused by any of the foregoing, will not be covered by ResMed's limited
warranty.
• Leave the humidifier tub to cool for ten minutes before handling to allow the water to cool and to
make sure that the humidifier tub is not too hot to touch.
• Only clean, maintain and/or reprocess the device and components according to the instructions
shown in this guide.
The following sections will help you with:
• Disassembling
• Cleaning
• Checking
• Reassembling.

English 25
Disassembling
1. Hold the humidifier tub at the top and bottom, press it gently and pull it away from the device.
Note: take care when handling the humidifier tub as the humidifier tub may be hot. Allow 10 minutes
for the heater plate and any excess water to cool.
2. Open the humidifier tub and discard any remaining water.
3. Pinch the cuff of the air tubing, and gently pull it away from the device.
4. Hold both the cuff of the air tubing and the swivel of the mask, then gently pull apart.
5. Locate the outlet connector on the inside of the device and release it by pressing the clip firmly.
6. Remove the outlet connector by pulling it out through the outlet connector socket at the rear of the
device.
Cleaning
The following instructions are for home cleaning.
You should clean the device, humidifier tub, air tubing, and outlet connector as described. For cleaning
your mask, refer to the mask user guide for detailed instructions.
Daily:
1. Empty the humidifier tub and wipe it thoroughly with a clean disposable cloth. Allow it to dry out of
direct sunlight.
2. Refill the humidifier tub with distilled water.
Weekly:
1. Wash the components using one of the following options:
• Wash the humidifier tub, air tubing and outlet connector in warm water using a household
dishwashing liquid. Components should not be washed in temperatures higher than 149°F (65°C)
OR
• Wash the humidifier tub and outlet connector in a solution of 1 part vinegar and 9 parts water. Wash
the air tubing in warm water using a household dishwashing liquid. The air tubing should not be
washed in temperatures higher than 149°F (65°C).
2. Rinse each component thoroughly in water.
3. Allow to dry out of direct sunlight or heat
4. Wipe the exterior of the device with a dry cloth.
Notes:
• The humidifier tub may be washed in a dishwasher on the delicate cycle (top shelf only).
• Do not wash the heated air tubing in a dishwasher or washing machine.
• The air filter is not washable or reusable.

26
Checking
WARNING
• Discontinue use and contact your care provider or ResMed Service Center if any of the following
occur:
• device does not perform as usual
• device is making unusual sounds
• device is damaged
• If using a bacterial/viral filter, regularly check it for signs of moisture or other contaminants,
particularly during nebulization or humidification. Failure to do so could result in increased
breathing resistance.
CAUTION
If any visible deterioration of a system component is apparent (cracking, discoloration, tears etc.),
the component should be discarded and replaced.
Regularly check the humidifier tub, air tubing, and air filter for any damage.
1. Check the humidifier tub:
• Replace it if it is leaking or has become cracked, cloudy, or pitted.
• Replace it if the seal is cracked or torn.
• Remove any white powder deposits using a solution of one-part household vinegar to 10 parts
water. Rinse with clean water.
2. Check the air tubing and replace it if there are any holes, tears, or cracks.
3. Check the air filter and replace it every six months. Replace it more often if there are any holes or
blockages by dirt or dust.
Replacing the air filter
1. Open the air filter cover and remove the old air filter.
2. Place a new air filter onto the air filter cover and then close the cover. Make sure the air filter and air
filter cover is fitted at all times to prevent water and dust from entering the device.
Note: The air filter is not washable or reusable.
Reassembling
When the humidifier tub and air tubing are dry, you can reassemble the parts.
To reassemble the AirSense 11 system:
1. Hold the outlet connector with the seal pointing to the left and the clip pointing forward.
2. Make sure the outlet connector is correctly aligned and insert the outlet connector into the socket.
3. Check the outlet connector is inserted correctly.
4. Connect the air tubing firmly to the air outlet located on the rear of the device.
5. Open the humidifier tub and fill it with distilled water under room temperature up to the maximum
water level mark.
6. Close the humidifier tub and insert it into the side of the device.
7. Connect the free end of the air tubing firmly onto the assembled mask.

English 27
Preparing the device for use between patients
When the device is used for multiple patients, for example, in a sleep lab, clinic, hospital or at a health care
provider, the outlet connector, air tubing , cleanable humidifier tub and device enclosure should be
reprocessed between each patient.
Described here are ResMed's recommended and validated procedures for cleaning and disinfecting the
outlet connector, air tubing, cleanable humidifier tub and device enclosure. The person executing
reprocessing activities is responsible for ensuring that reprocessing is completed in line with ResMed's
validated procedures. Components not identified in the reprocessing instructions do not require
reprocessing or are intended for single patient use.
Note: The standard HumidAir 11 tub cannot be reprocessed.
WARNING
• Always follow cleaning and disinfection instructions. Some cleaning products may damage the
components and their function or leave harmful residual vapors.
• Any deviations from the procedures or claimed maximum number of cycles in this guide can
have an adverse effect on the components and consequently the safety or the quality of therapy.
• When using detergents, disinfectants or equipment, always follow the instructions provided by
the manufacturer of those products. In the event of conflict, this guide takes precedence.
• Always follow safe operating practices, including the use of appropriate Personal Protective
Equipment (PPE), as required. Refer to the instructions provided by the manufacturer of those
products for more details.
• Beware of electrocution:
• Do not immerse the device, AC Adaptor or power cord in water.
• Do not connect to power while the device is wet. Make sure that all parts are dry before
plugging it in.
General summary
ResMed has validated the following number of cycles for cleaning and disinfection using a manual cleaning
method.
Components
Cleaning - Mild alkaline, anionic detergent (eg, Alconox)
System component Chemical high level disinfection
eg, CIDEX-OPA
Thermal high level disinfection
167ºF (75ºC) for 30 minutes
Outlet connector 120 80
HumidAir 11 Cleanable tub 120 80
ClimateLineAir 11 30 30
Standard tubing 30 30
SlimLine 30 30
Device
Cleaning and low level disinfection
Device Enclosure CaviWipes1™

28
Disassembling
Before disassembly, turn off the device and ensure the AC adapter has been removed.
Cleanable Humidifier tub
1. Hold the humidifier tub at the top and bottom, press it gently and pull it away from the device.
Note: take care when handling the humidifier tub as the humidifier tub may be hot. Allow 10 minutes
for the heater plate and any excess water to cool.
2. Hold the base of the humidifier tub and fully open the humidifier tub lid and pull it away so that it easily
detaches from the base.
Air tubing
1. Pinch the cuff of the air tubing, and gently pull it away from the device.
2. Hold both the cuff of the air tubing and the swivel of the mask, then gently pull apart.
Outlet connector
1. Locate the outlet connector on the inside of the device and release it by pressing the clip firmly.
2. Remove the outlet connector by pulling it out through the outlet connector socket at the rear of the
device.

English 29
Device enclosure
Cleaning
Clean the device enclosure using an alcohol based cleaning and disinfection wipe. ResMed has validated
CaviWipes1.
1. Wipe the exterior of the device using a wipe until visually clean following the manufacturer's instruction
for cleaning. Use a minimum of two wipes.
If visual debris is still present, perform the following:
2. Clean the exterior of the device with a dry, soft bristle brush and wipe the exterior of the device using a
new cleaning and disinfection wipe following the manufacturer's instruction for cleaning.
Disinfection
Repeat the first step with a new wipe and follow the manufacturer's instructions for low level disinfection.
Note: Failure to clean the component as indicated may result in inadequate disinfection.
Drying
Allow sufficient time for the device to air dry completely.
Note: Drying is not required after cleaning if disinfection is continued immediately.
Inspection
Perform a visual inspection of the device casing. If any visible deterioration is apparent (cracking, crazing
etc) discontinue use and contact your care provider or your ResMed Service Centre.
Air tubing, outlet connector and HumidAir 11 tub
Cleaning
1. Make a solution of a mild alkaline anionic detergent and water
1
as directed by the manufacturer's
instructions. ResMed has validated :
• Alconox™ at 1% (10 g/L) in water
1
at 69.8ºF to 131ºF (21ºC to 55ºC)
2. Soak all components for 5-10 minutes. Agitate the component in the cleaning solution to ensure there
are no air bubbles.
3. Clean the inside and outside of all components with a soft bristle brush while soaking in a detergent
solution. Pay particular attention to all crevices and cavities.
• Tubing (Standard, SlimLine and ClimateLineAir 11): 3 minutes of brushing
Note: A soft bristle tube/bottle brush is required to clean the inside of the tubing. Remove tubes from
the detergent solution to assist brushing.
• Outlet connector: 1 minute of brushing
• HumidAir 11 Cleanable tub: 2 minutes of brushing
4. Thoroughly rinse each component as follows: in 5 liters of water
1
at ≤ 140ºF (≤ 60ºC) for each
component by immersing it. Rinse tubing for 30-60 seconds. Agitate the component in the rinsing
water to ensure there are no air bubbles.
5. Repeat the rinse procedure two additional times using fresh water
1
for a total of three rinses.
Note: Failure to clean the component as indicated may result in inadequate disinfection.
Inspection
Inspect and if required, repeat the cleaning steps until visually clean. Shake air tubing to remove excess
water.
Drying
Allow the components to dry out of direct sunlight.
Note: Drying is not required after cleaning if thermal disinfection is continued immediately.
1
For all cleaning, rinsing and disinfection steps use drinking quality water.
30
Disinfection
High Level disinfection
In the following procedures, only one disinfection process needs to be performed: Thermal disinfection OR
Chemical disinfection.
High level thermal disinfection
1. Immerse the components in a water
2
bath. Agitate the components in the water bath to ensure no air
bubbles are trapped.
2. Soak the components in a hot water bath. ResMed has validated:
• Water bath: at 167ºF (75ºC) for 30 minutes
Note: Higher temperatures may damage the components.
3. Allow the components to dry out of direct sunlight.
OR
High level chemical disinfection
1. Make a solution of Ortho-phthalaldehyde 0.55% as directed by the disinfectant manufacturer. ResMed
has validated:
• CIDEX® OPA Ortho-phthalaldehyde
2. Soak the components in the solution at room temperature (approximately 69.8ºF to 77ºF (21ºC to 25ºC)
for 12 minutes. Agitate the components in the disinfection solution to ensure there are no air bubbles.
3. Rinse and agitate the components in water
2
5 liters per component at
≤ 140ºF (≤60ºC) for 1 minute. Shake air tubing to remove excess water.
Repeat the rinse procedure two additional times using fresh water
2
for a total of three rinses.
1. Allow the components to dry out of direct sunlight.
Inspection
Perform a visual inspection of each component.If any visible deterioration is apparent (holes, tears or
cracks etc) replace the component.
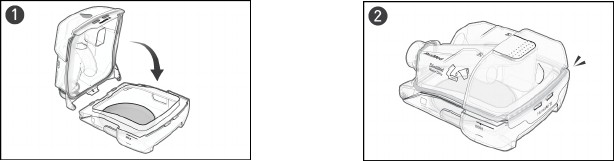
Reassembling
Once the components are dry, reassemble the device.
Outlet connector
1. Hold the outlet connector with the seal pointing to the left and the clip pointing forward.
2. Make sure the outlet connector is correctly aligned and insert the outlet connector into the socket. It
will click in place.
2
For all cleaning, rinsing and disinfection steps use drinking quality water.
English 31
Cleanable HumidAir 11 tub
1. Insert one side of the lid into the pivot hole of the base. Insert the other side of the lid into the pivot
hole.
2. Push the lid down until it clicks in place.
Air tubing
1. Connect the air tubing firmly to the air outlet located on the rear of the device.
2. Connect the free end of the air tubing firmly onto the assembled mask.
Packing and storing
Store in a dry dust-free environment away from direct sunlight.
Storage and transport temperature: -13°F to +158°F (-25°C to +70°C)
Storage and transport humidity: 5 to 95% relative humidity, non-condensing

32
Data management and therapy compliance
For therapy management, the AirSense 11 device stores patient therapy data on the device and may have
the ability to transfer it remotely to the care provider if wireless network is available. Data can then be
accessed via ResMed's AirView
™
therapy management solution.
The AirSense 11 device also stores data on the SD card. This data can be transferred via an SD Card
Reader to ResMed's ResScan
™
therapy management system. The SD card should not be used for any
other purpose as it may corrupt therapy data stored on the card. Do not remove the SD card from the
device when the SD light is flashing, because data is being written to the card.
For more information on therapy management with AirView or ResScan, refer to the manuals supplied
with the software.
Remote monitoring
The AirSense 11 device has cellular communication which has the ability to automatically transmit
summary and detailed data on a regular basis. It also allows you to change settings remotely.
The Wireless signal strength icon
displayed at the top right of the screen indicates the signal strength.
Advise the patient to check the signal strength on their device.
Therapy data might not be transmitted if used outside of the country or region of purchase.
For AirSense 11 devices that are bundled with an SD card, they will already be inserted and ready to use.
Once the data is loaded into ResScan or AirView via the SD Card Reader, you can review and analyze data,
as well as update therapy settings and transfer them to the patient’s device via the SD card.
To remove the SD card cover and insert SD card:
1. Push the SD card cover.
2. Remove the SD card cover and keep the SD card cover in a safe place.
3. Insert the SD card.
4. Push in the SD card until it clicks in place.
To remove the SD card:
1. Push in the SD card to release it.
2. Place the SD card in the protective folder and follow your care provider’s instructions.

English 33
Data storage
The AirSense 11 device stores summary data such as AHI, Total Hours Used and Leak. Detailed data such
as snore is stored on the SD card and can be viewed with AirView and ResScan. High resolution flow and
pressure data are stored on the SD card.
Data can be transmitted to therapy management software either remotely via cellular communication, or
via SD card. The different ways of transmitting data are detailed in the table below.
For more information on therapy management with AirView or ResScan, refer to the manuals supplied
with the software.
Type of data
Transmission method
Sessions stored
Cellular
communication
to AirView
SD card to
ResScan
SD Card to
AirView
(card-to-
cloud)
Summary data (compliance data)
365
Detailed data
Limited by usage and SD
card storage capacity
High resolution flow (25 Hz - every 40 ms)
Detailed data is stored on the SD card and can be viewed via ResScan or AirView. Examples of detailed
data available is shown below.
Detailed data
Parameter
Sampling rate
ResScan
AirView
Apnea or hypopnea events aperiodic aperiodic
CSR aperiodic aperiodic
RERA (AirSense 11 Elite only) aperiodic aperiodic
Flow limitation (flat to round) 1/2 Hz (2 sec) 1 min
Leak (L/sec) 1/2 Hz (2 sec) 1 min
Minute ventilation (L/min) 1/2 Hz (2 sec) 1 min
Pressure (cm H
2
O)/hPa) 1/2 Hz (2 sec) 1 min
Snore (quiet to loud) 1/2 Hz (2 sec) 1 min

34
Troubleshooting
If there is a problem, try the following suggestions. If you are not able to fix the problem, contact your local
ResMed dealer or ResMed office. Do not open the device.
General troubleshooting
Problem/possible cause
Solution
Air is leaking from around the mask
Mask may be fitted incorrectly. Make sure the mask is fitted correctly. See the mask user guide for fitting
instructions or run the Mask Fit function.
The patient is getting a dry or blocked nose
Humidity level may be set too low.
Increase the Humidity Level.
There are droplets of water in the mask and air tubing
Humidity level may be set too high.
Decrease the Humidity Level.
Tube temperature may be too low
Increase the Tube temp
The patient is getting a very dry mouth
Air may be escaping through the patient's mouth.
Increase the Humidity Level.
The patient may need a chin strap to keep the mouth closed or a full face
mask.
The patient feels that too much air is being delivered from the device
Ramp may be turned off
Use the Ramp Time option.
The patient feels that not enough air is being delivered from the device
Ramp may be in progress
Wait for air pressure to build up or turn Ramp Time off
Ramp start pressure may be too low Increase Ramp start pressure.
No display
Backlight on the screen may have turned off. It turns
off automatically after a short period of time
Press the Start therapy /standby button located at the top of the device or
touch the screen.
Power may not be connected. Connect the AC adaptor and make sure the plug is fully inserted.
Therapy has stopped but the device is still blowing air
Device is cooling down
Device blows a small amount of air in order to avoid condensation in the
air tubing. it will stop automatically after 30 minutes.
Humidifier tub is leaking
Humidifier tub may not be assembled correctly. Check for damage and reassemble the humidifier tub correctly.
Humidifier tub may be damaged or cracked. Replace the humidifier tub.
The patient is not getting enough air/oxygen flow is disrupted
Tubing or humidifier tub may be blocked Check for blockages. Reconnect the tubing and reassemble the humidifier
tub correctly.
The patient's therapy data has not been transmitted
Wireless coverage may be poor. Advise the patient to place the device where there is coverage (ie, on
their bedside table, not in a drawer or on the floor).
The wireless signal strength icon
indicates good coverage when all
bars are displayed, and poor coverage when fewer bars are displayed.

English 35
Problem/possible cause
Solution
The No wireless connection icon is displayed on the
top right of the screen. No wireless network available.
Advise the patient that therapy data can be sent via SD Card.
Device may be in Airplane Mode.
Turn off Airplane Mode.
For instructions see the User Guide.
SmartStart is enabled, but the device does not automatically start when the patient breathes into their mask
Breath is not deep enough to trigger SmartStart To start therapy, take a deep breath in and out through the mask, before
breathing normally.
Press the Start therapy/Standby button located on the top of the device
There is excessive leak Adjust the mask and headgear
Air Tubing may not be connected properly. Connect firmly at both ends.
SmartStop is enabled but does not automatically stop when the patient removes their mask
Incompatible mask being used Only use equipment recommended by ResMed.
Contact ResMed or see ResMed.com for more information
If the patient is using a nasal pillows mask with set pressure less than
7cm H
2
O (7 hPa), SmartStop will not work and should be disabled.
If the patient is using a conduit mask, SmartStop will not work and should
be disabled.
Device error messages
Device message/possible cause Solution
High leak detected, check your humidifier or side cover
Humidifier tub may not be inserted properly. Make sure the humidifier tub is correctly inserted.
High leak detected, connect your tubing
Air tubing may not be connected properly. Make sure the air tubing is firmly connected at both ends.
Mask may be fitted incorrectly. Make sure the mask is fitted correctly. See the mask user guide for
fitting instructions or use the Mask Fit function to check the mask fit
and seal.
Tubing blocked, check your tubing
Air tubing may be blocked.
Check the air tubing and remove any blockages. Press the dial to
clear the message and then press Start therapy/ Standby button to
restart the device.
Read only card, please remove, unlock and re-insert SD card
SD card switch may be in the lock (read-only) position.
Move the switch on the SD card from the lock position
to the
unlock position
and then re-insert it.
System fault, refer to user guide, Error 4
Device may have been left in a hot environment. Allow to cool before re-use. Disconnect the AC adaptor and then
reconnect it to restart the device.
Air filter may be blocked. Check the air filter and replace it if there are any blockages.
Disconnect the AC adaptor and then reconnect it to restart the
device.

36
Device message/possible cause Solution
Air tubing may be blocked.
Check the air tubing and remove any blockages. Press the dial to
clear the message and then press Start therapy/Standby button to
restart the device.
There may be water in the air tubing. Empty the water from the air tubing. Disconnect the AC adaptor and
then reconnect it to restart the device.
All other error messages, for example, System fault, refer to user guide, Error XX
An unrecoverable error has occurred on the device. Contact your local ResMed dealer or ResMed office. Do not open the
device.
General Warnings
WARNING
• Any change or modification to the product is not expressly approved by ResMed and could void
the user's authority to operate the device.
• The device has not been tested or certified for use in the vicinity of X-ray, CT or MRI equipment.
Do not bring the device within 13 ft (4 m) of X-ray or CT equipment. Never bring the device into
an MR (Magnetic Resonance) environment.
• This medical device uses a small bore connector design that is different to those specified in
ISO80369-2. It may be possible to connect this device with other medical devices (eg, devices that
deliver fluid or medicine) which may result in a hazardous situation and cause harm to the
patient. Extra care should be taken by the user to mitigate any risks that may result.
• The use of accessories other than those specified for the device is not recommended. These may
increase radio frequency energy or be influenced by the interference and result in improper
operation.
• The device should not be used adjacent to or stacked with other equipment. If adjacent or
stacked use is necessary, the device should be observed to verify normal operation in the
configuration in which it will be used.
• Do not use the device with water in the humidifier tub while in transit (eg, on a plane or vehicle)
due to the risk of:
• water spilling into the device
• the inhalation of water during turbulence.
• Make sure that the humidifier tub is empty before transporting the device.
For any serious incidents that occur in relation to this device, these should be reported to ResMed and the
competent authority in your country.

English 37
Technical specifications
Operating pressure range
4 to 20 cm
H
2
O (4 to 20 hPa)
Maximum single fault steady state pressure
Device will shut down in the presence of a single fault if the steady state pressure exceeds:
40 cm H
2
O (40 hPa) for more than 1 second.
Pressure measurement tolerance
± 0.5 cm H
2
O (0.5 hPa) ±4% of measured reading
Flow measurement tolerance
± 6 L/min or 10% of reading, whichever is greater, at 0 to 150 L/min positive flow
Mode pressure ranges
CPAP: 4-20 cm H
2
O (4-20 hPa) (measured at the mask)
CPAP with EPR mode:
4-20 cm H
2
O (4-20 hPa) CPAP with EPR settings: EPR off, Level 1 = 1.0 cm H
2
O (1 hPa), Level 2 = 2.0 cm H
2
O
(2 hPa), Level 3 = 3.0 cm H
2
O (3 hPa).
AutoSet, AutoSet for Her mode: 4-20 cm H
2
O (4-20 hPa)
AutoSet, AutoSet for Her mode with EPR:
4-20 cm H
2
O (4-20 hPa) APAP with EPR settings: EPR off, Level 1 = 1.0 cm H
2
O (1 hPa),
Level 2 = 2.0 cm H
2
O (2 hPa), Level 3 = 3.0 cm H
2
O (3 hPa).
EPR reduces the pressure during expiration by the amount dependent on the level set above, but the pressure delivered will not
drop below 4.0 cm H
2
O (4 hPa).
Flow (maximum) at set pressures
The following are measured according to ISO 80601-2-70 201.12.1.103:
Pressure
AirSense 11, humidifier tub
and Standard air tubing
AirSense 11, humidifier tub
and SlimLine
AirSense 11, humidifier tub
and ClimateLineAir 11
cm H
2
O (hPa) L/min L/min L/min
4 150 145 144
8
147
142
141
12
143
138
138
16
140
135
134
20 136 131 129
Note: Refer to the relevant measurement uncertainty from the Measurement system uncertainties table.
Sound
Declared dual-number noise emission values in accordance with ISO 4871:1996
Sound pressure level measured according to ISO 80601-2-70:2015 (CPAP mode):
Device with SlimLine and humidification 27 dBA with uncertainty of 2 dBA
Sound power level measured according to ISO 80601-2-70:2015 (CPAP mode):
Device with SlimLine and humidification
35 dBA with uncertainty of 2 dBA
Device and humidifier tub
Dimensions (H x W x D): 3.72" x 10.21" x 5.45"
(94.5 mm x 259.4 mm x 138.5 mm)
Air outlet:
The 22 mm conical outlet connector complies with EN ISO 5356-1:2015
Weight (device and standard humidifier tub): 40 oz (1130 g)
Housing construction:
Flame retardant engineering thermoplastic
Hot plate - Material: Stainless steel
Water capacity:
380 mL
Time between each refill of the humidifier tub: > 8 hours ±0.5 hours (tested at 23 ±2°C / 73.4 ± 3.6 °F)
Recommended water type to use in the humidifier tub
(Standard tub):
Distilled water (Americas only)

38
Humidifier tub - Material: Injection molded plastic, stainless steel and silicone seal
65W power supply unit
AC input range
100-240v, 50-60Hz, 2.0A
115V, 400Hz, 1.5A (for aircraft use)
DC output 24 VDC ± 1 VDC, 2.71A
Typical power consumption
56.1W (111.5VA)
Peak power consumption 73.2W (137.6VA)
Class of equipment
Class ll
Environmental conditions
Operating temperature +41°F to +95°F (+5°C to +35°C)
Note:
The airflow for breathing produced by this therapy device can be
higher than the room temperature. Under extreme ambient temperature
conditions (104°F/40°C) the device remains safe.
Operating humidity 10 to 95% relative humidity, non-condensing
Operating altitude
Sea level to 9,870' (3,010 m); air pressure range 1060 hPa to 700 hPa
Storage pressure/Storage altitude 1060 to 700 hPa relative humidity, non-condensing
Storage and transport temperature
-13°F to +158°F (-25°C to +70°C)
Storage and transport humidity 5 to 95% relative humidity, non-condensing
Air Filter
Standard:
Material: Polyester non woven fiber
Average arrestance: >75%, when tested to EN779.
Hypoallergenic: Material: Blended synthetic fibers in a polypropylene carrier
Efficiency: >80% (average) when tested to EN13274-7.
Note: The use of a ResMed approved hypoallergenic filter will result in a
small reduction in the accuracy of the delivered pressure at high leaks.
Electromagnetic compatibility
The AirSense 11 complies with all applicable electromagnetic compatibility requirements (EMC) according to IEC 60601-1-2:2014,
for residential, commercial and light industry environments.
Portable and mobile RF communications equipment should be used no closer to any part of the machine, including cables, than the
recommended 3.94" (10 cm) separation distance.
The AirSense 11 has been designed to meet EMC standards. However, should you suspect that the device performance (eg.
pressure or flow) is affected by other equipment, move the device away from the possible cause of interference.
Information regarding the electromagnetic emissions and immunity of this ResMed device can be found in
ResMed.com/downloads/devices.
IEC 60601-1 (Edition 3.1) classification
Class II (double insulation), Type BF, Ingress protection IP22.
Supplemental oxygen maximum flow
15 L/min
Aircraft use
ResMed confirms that the machine meets the Federal Aviation Administration (FAA) requirements (RTCA/DO-160, section 21,
category M; RTCA-DO-160, section 20, category T) for all phases of air travel.
Design life
Device, power supply unit: 5 years
Standard humidifier tub:
6 months
Air tubing: 6 months
General
The patient is an intended operator.

English 39
Pneumatic flow path
1.
Flow sensor
2. Blower
3. Pressure sensor
4. Mask
5. Air tubing
6. Bacterial/viral filter
7. Humidifier
8. Device
9. Inlet filter
Displayed values
Value
Range
Accuracy
Display resolution
Pressure at mask:
Displayed mask pressure
1
4-20 cm H
2
O (4-20 hPa) ±0.5 cm H
2
O (0.5 hPa) ±4% of
measured reading
0.1 cm H
2
O (0.1 hPa)
Flow derived values:
Leak
1
0-120 L/min ± 12 L/min or 20% of reading
whichever is greater, 0 to 60
L/min
1 L/min
1
Results may be inaccurate in the presence of leaks or supplemental oxygen
Pressure accuracy
Maximum static pressure variation at 10 cm H
2
O (10 hPa) according to ISO 80601-2-70:2015
Device with humidifier tub and air tubing:
±0.5 cm H
2
O (±0.5 hPa)
Note: Refer to the relevant measurement uncertainty from the Measurement system uncertainties table.
Maximum dynamic pressure variation according to ISO 80601-2-70:2015
AirSense 11 with humidifier tub and air tubing
Breath rate 10 BPM 15 BPM 20 BPM
Dynamic pressure variation (cmH
2
O [hPa])
0.5
0.5
0.8
Measurement system uncertainties
In accordance with ISO 80601-2-70:2015 the measurement uncertainty of the manufacturer's test equipment is:
For measures of flow: ± 3.9 L/min
For measures of static pressure:
± 0.15 cm H
2
O (± 0.15hPa)
For measures of dynamic pressure: ± 0.04 cm H
2
O (± 0.04hPa)
Note: ISO 80601-2-70:2015 stated accuracies and test results provided in this manual for these items already include the relevant
measurement uncertainty from the table above.
In accordance with ISO 80601-2-74:2017 the measurement uncertainty of the manufacturer's test equipment is
For measures of humidification output
± 0.5 mg/L BTPS
Bluetooth
Technology used:
Bluetooth Low Energy (BLE)
Connection types: GATT
Frequency: 2400 to 2483.5 MHz
Max RF power output:
+4 dBm
Operation range:
10 m (Class 2)
Wireless module
Bluetooth
Technology used:
Bluetooth Low Energy (BLE)
Connection types: GATT

40
Frequency: 2400 to 2483.5 MHz
Max RF power output:
+4 dBm
Operation range: 10 m (Class 2)
Cellular module
Technology used:
Frequencies (MHz)
Max RF power output (dBm)
2G 900/ 1800 * 33.0
3G
850/ 900/ 1700/ 1900/ 2100*
23.5
4G LTE Cat 1 700/ 850/ 1700/ 1900* 23.0
*Bands may not be available in all regions.
FCC ID: 2ACHL-AIR114G
IC: 9103A-AIR114G
The AirSense 11 device complies with FCC Rules and Industry Canada rules. Operation is subject to the following two conditions:
1. This device may not cause harmful interference; and
2. This device must accept any interference received, including interference that may cause undesired operation.
The AirSense 11 device should be installed and operated with minimum distance of 0.59" (15 mm) between the equipment and the
user's body.
Additional information regarding the FCC Rules and IC compliance for this device can be found on ResMed.com/downloads/devices
In Canada, the device has been designed to comply with safety standards to radio waves (SAR) in accordance to RSS-102.
Humidifier
Maximum heater plate temperature: 154ºF (68ºC)
Temperature cut-out (heater):
165ºF (74ºC)
Maximum gas temperature (at mask)
1
: ≤ 106ºF (41ºC)
1
The air flow for breathing produced by this therapy device can be higher than the temperature of the room. Under extreme
ambient temperature conditions (104ºF/40ºC) the device remains safe.
Humidifier performance
SlimLine/Standard tubing
Mask Pressure
cm H
2
O (hPa)
Nominal RH output % at 72ºF (22ºC) ambient
temperature
Nominal system output mg/L AH
1
, BTPS
2
Setting 4 (default
setting)
Setting 8 (maximum
setting)
Setting 4 (default
setting)
Setting 8
3
(maximum
setting)
4
80%
100%
≥6
>12
10 80% 100% ≥6 >12
20
80%
100%
≥6
>12
Climate Control Auto - ClimateLineAir 11
Mask Pressure
cm H
2
O (hPa)
Nominal RH output % at 72ºF (22ºC) ambient
temperature
Nominal system output mg/L AH
1
, BTPS
2
4
85%
≥ 12
10 85% ≥ 12
20
85%
≥ 12
1
AH - Absolute Humidity in mg/L
2
BTPS - Body Temperature Pressure Saturated
3
Humidifier performance meets ISO 80601-2-74:2017 performance > 12 mg/L BTPS tested at 59°F to 95°F (15°C to 35°C)

English 41
Air tubing
ClimateLineAir 11 SlimLine/ Standard
ClimateLineAir 11 temperature range 60 to 86ºF (16 to 30ºC) -
ClimateLineAir 11 temperature cut out
≤106ºF (≤41ºC)
-
Maximum recommended pressure 30 cm H
2
O (30 hPa) 30 cm H
2
O (30 hPa)
Maximum working temperature, when used with a
humidifier
- ≤106ºF (≤41ºC)
Material Flexible plastic and electrical
components
Flexible plastic
Inner diameter 0.6" (15 mm) SlimLine: 0.6" (15 mm)
Standard: 0.74" (19 mm)
Length 6'6" (2.0 m) SlimLine: 6' (1.8 m)
Standard: 6'6" (2.0 m)
Note: The manufacturer reserves the right to change these specifications without notice.
Air tubing resistance to flow and compliance information
Refer to the Air tubing compliance guide in ResMed.com.
Characteristics of compatible Bacterial/ Viral (B/V) filters
Resistance over the flow range:
Recommend a B/V filter with resistance of < 2.5cm H
2
O at 60 L/min
Dead space (volume): < 90 mL
Connectors:
ISO 5356-1:2015 compliant connectors
Bacterial Filtration Efficiency (ie BFE): >99.9%
Viral Filtration Efficiency (ie VFE):
>99.7%
Maximum duration of use: Refer to manufacturer's datasheet
Replacing B/V filter:
Refer to manufacturer's datasheet
Compliance: < 0.103mL/ cm H
2
O (<0.103mL/hPa)
Note: B/V filters are high in impedance and show variability in their pneumatic characteristics that may affect delivered
pressure and the accuracy of displayed and reported values
Applied parts
Patient interface (mask) and air tubing
Symbols
Follow instructions before use. Indicates a warning or caution. Temperature limitation.
Humidity limitation. Operating altitude. Atmospheric pressure limitation. Manufacturer.
Direct current. Class II equipment. Protected against finger sized objects and against
dripping water when tilted up to 15 degrees from specified orientation.
Non-ionising radiation. MR
unsafe (do not use in the vicinity of an MRI device).
RTCA/DO-160 Section 21, Category M Compliant &
FAA Compliant.
Type BF applied part. Date of Manufacture Medical device. Catalog
number.
Device number. Serial number. Batch code. European Authorized
Representative.
Bluetooth. Start therapy/Standby. Prescription only (In the US, Federal
law restricts these devices to sale by or on the order of a physician).
Use distilled water only.
Maximum water level. Open tub to fill.
See symbols glossary at ResMed.com/symbols.

42
Environmental information
This device should be disposed of separately, not as unsorted municipal waste. To dispose of your device,
you should use appropriate collection, reuse and recycling systems available in your region. The use of
these collection, reuse and recycling systems is designed to reduce pressure on natural resources and
prevent hazardous substances from damaging the environment.
If you need information on these disposal systems, please contact your local waste administration. The
crossed-bin symbol invites you to use these disposal systems. If you require information on collection and
disposal of your ResMed device please contact your ResMed office, local distributor or go to
ResMed.com/environment.
California Perchlorate Information:
The coin-cell battery within this device may contain Perchlorate Material - special handling may apply.
See: www.dtsc.ca.gov/hazardouswaste/perchlorate
Limited warranty
ResMed Pty Ltd (hereafter 'ResMed') warrants that your ResMed product shall be free from defects in
material and workmanship from the date of purchase for the period specified below.
Product Warranty period
• Mask systems (including mask frame, cushion, headgear and tubing)—excluding single-
use devices
• Accessories—excluding single-use devices
• Flex-type finger pulse sensors
• Humidifier water tubs
90 days
•
Batteries for use in ResMed internal and external battery systems
6 months
• Clip-type finger pulse sensors
• CPAP and bilevel device data modules
• Oximeters and CPAP and bilevel device oximeter adapters
• Humidifiers and humidifier cleanable water tubs
• Titration control devices
1 year
•
CPAP, bilevel and ventilation devices (including external power supply units)
• Battery accessories
• Portable diagnostic/screening devices
2 years
This warranty is only available to the initial consumer. It is not transferable.
During the warranty period, if the product fails under conditions of normal use, ResMed will repair or
replace, at its option, the defective product or any of its components.
This limited warranty does not cover: a) any damage caused as a result of improper use, abuse,
modification or alteration of the product; b) repairs carried out by any service organization that has not been
expressly authorized by ResMed to perform such repairs; c) any damage or contamination due to cigarette,
pipe, cigar or other smoke; and d) any damage caused by exposure to ozone, activated oxygen or other
gasses.
Warranty is void on product sold, or resold, outside the region of original purchase.
Warranty claims on defective product must be made by the initial consumer at the point of purchase.
This warranty replaces all other expressed or implied warranties, including any implied warranty of
merchantability or fitness for a particular purpose. Some regions or states do not allow limitations on how
long an implied warranty lasts, so the above limitation may not apply to you.
ResMed shall not be responsible for any incidental or consequential damages claimed to have resulted
from the sale, installation or use of any ResMed product. Some regions or states do not allow the
exclusion or limitation of incidental or consequential damages, so the above limitation may not apply to
you.
English 43
This warranty gives you specific legal rights, and you may also have other rights which vary from region to
region. For further information on your warranty rights, contact your local ResMed dealer or ResMed
office.
Visit ResMed.com for the latest information on ResMed's Limited Warranty.
Further information
If you require additional information on how to setup, use or maintain the Air11™ system (including
ClimateLineAir 11 heated tubing), or to report unexpected operation or events, please contact the ResMed
Service Centre or your care provider.

ResMed Pty Ltd
1 Elizabeth Macarthur Drive Bella Vista NSW 2153 Australia
S
ee ResMed.com for other ResMed locations worldwide. Air11, AirSense, AirView, AutoSet, ClimateLineAir, HumidAir, myAir, SlimLine, EPR,
ResScan and SmartStart are trademarks and/or registered trademarks of the ResMed family of companies. For patent and other intellectual property
information, see ResMed.com/ip. SD Logo is a trademark of SD-3C, LLC. Google Play and the Google Play logo are trademarks of Google LLC. Apple
and the Apple logo are trademarks of Apple Inc., registered in the U.S and other countries. App Store is a service mark of Apple Inc., registered in the
U.S and other countries. CaviWipes1 is a registered trademark of Metrex Research, LLC. Alconox is a trademark of Alconox INC. CIDEX OPA is a
trademark of ASP Global Manufacturing GmbH. The Bluetooth
®
word mark and logos are registered trademarks owned by Bluetooth SIG, Inc. and any
use of such marks by ResMed is under license. © 2021 ResMed. 398230/2 2021-06
ResMed.com
