
______________________________________________________________________________________
Laboratory & Research
Safety Plan:
1. General Safety Plan
2. Waste Management Logbook
3. Rules and Procedures for the Use of Radioactive
Material at The Pennsylvania State University
4. Unit Specific Plan
______________________________________________________________________________________
The Pennsylvania State University
Electronic version: www.ehs. psu.edu
Wiki site: https://wikispaces.psu.edu/spaces/listattachmentsforspace.action?key=SOP
2
TABLE OF CONTENTS
1.0 INTRODUCTION
2.0 RESPONSIBILITIES
2.1 Penn State Leadership
2.2 Senior Vice President for Finance and Business
2.3 Associate Vice President of Office of Physical Plant
2.4 Environmental Health and Safety
2.5 Budget Executives and Budget Administrators
2.6 University Safety Council
2.7 The Institutional Biosafety Committee
2.8 Department Heads, Center Directors, and Other Facility Directors
2.9 Principal Investigators and Supervisors
2.10 Laboratory/Research Safety Officer
2.11 The Individual
2.12 Laws and Regulations
2.13 Students
3.0 THE LABORATORY AND RESEARCH SAFETY PROGRAM
3.1 Laboratory and Research Safety Plan
3.2 Unit Specific Plan
3.2.1 Initial Preparation
3.2.2 Change of Facility
3.2.3 Addition of or Change to a Specific Project
3.3 Inspections
3
GENERAL SAFETY PLAN
4.0 UNIVERSITY EMERGENCY INFORMATION
4.1 Where to Find Specific Information
4.2 University Emergency Response Plan
4.3 Building Emergency and Evacuation Plans
4.4 Power Failure
4.5 Incident (Accident) Reporting
4.6 EHS Assistance
4.7 Personal Injury
4.7.1 Burn
4.7.2 Inhalation
4.7.3 Ingestion
4.7.4 Puncture or Cut
4.7.5 Needle stick
5.0 GENERAL SAFETY
5.1 Personal Hygiene
5.2 Personal Protective Clothing and Equipment
5.2.1 Clothing
5.2.2 Eye Protection
5.2.3 Gloves
5.2.4 Respirators
5.3 General Laboratory Protocol
5.3.1 Housekeeping
5.3.2 Cleaning Glassware
5.3.3 Laboratory Animals
5.3.4 Relocating or Closing a Laboratory
5.3.5 Transportation of Hazardous Materials
5.3.6 Laboratory Doors
5.3.7 Children in Laboratories
5.4 General Laboratory Techniques
4
5.4.1 Laboratory Ventilation
5.4.2 Chemical Fume Hoods
5.4.3 Safety Showers
5.4.4 Eyewash Fountain
5.4.5
Pesticide Decontamination Supplies
5.4.6 Laboratory Sinks and Drain Traps
5.4.7 Electrical Equipment
5.4.8 Static Electricity
5.4.9 Centrifuges
5.4.10 Vacuum Pumps
5.4.11 Drying Ovens and Furnaces
5.4.12 Syringes and Scalpel Blades
5.4.13 Facility Cleaning and Maintenance
5.4.14 Glassware
5.4.15 Assembling Apparatus
5.4.16 Fire Extinguisher Policy
5.4.17 Special Precautions against Ultraviolet Light
5.5 Signs and Labels for Laboratories
5.6 Training
5.7 Machine Shop Equipment
6.0 CHEMICAL HAZARDS
6.1 Hazard Communication
6.1.1 Safety Data Sheets
6.1.2 Chemical Labels
6.2 Exposure to Chemicals
6.2.1 Exposure Routes
6.2.2 Acute and Chronic Toxicity
6.2.3 Carcinogenicity
6.2.4 Reproductive Toxins
6.2.5 Designated Area
6.2.6 Monitoring Airborne Concentrations of Contaminants
6.3 Guidelines for Handling Chemicals
6.3.1 General Guidelines
6.3.2 Guidelines for Working with Chemicals of Acute Toxicity
6.3.3 Guidelines for Working with Pesticides
6.3.4 Guidelines for Chemicals with High Chronic Toxicity, Carcinogens, and
Reproductive Toxins
5
6.4 Chemical Emergency Procedures
6.5 Medical Surveillance
6.5.1 When is Medical Surveillance Required?
6.5.2 Medical Consultation and Evaluation
6.5.3 Medical Surveillance for Chemicals of High Chronic Toxicity
6.6 Chemical Storage
6.6.1 Chemical Dating
6.6.2 Chemical Compatibility
6.6.3 Storage Facilities
6.6.4 Inspection of Stored Chemicals
6.6.5 Refrigerator Storage
6.6.6 Tanks and Drums
6.7 Safety for Specific Chemical Operations
6.7.1 Unattended/Overnight Operations
6.7.2 Procedures for Working Alone
6.7.3 Extractions and Distillations
6.7.4 Temperature Control
6.7.4.1 Oil and Sand Baths
6.7.4.2 Cooling Baths
6.7.5 Reduced Pressure Operations
6.7.6 Cold Traps
6.7.7 Transporting Chemicals In-House
6.8 Hazards of Chemical Groups
6.8.1 Corrosives: Acids and Bases
6.8.2 Flammable and Combustible Liquids
6.8.3 Compressed Gases
6.8.3.1 Acetylene
6.8.3.2 Lecture Bottles
6.8.4 Cryogenic Liquids and Liquefied Gases
6.8.5 Highly Reactive Chemicals
6.8.5.1 Organic Peroxides
6.8.5.2 Peroxide-Forming Chemicals
6.8.5.3 Polynitro Compounds
6.8.5.4 Catalysts
6.8.5.5 Calorimeters (Commonly Known as Parr Bombs)
6.8.5.6 Sodium Azide
6.8.5.7 Organometallics
6.8.5.8 Hydrides
6.9 Chemical Waste Management
6
6.9.1 Pickups
6.9.2 Sanitary Sewer Disposal
6.9.3 Treatment
6.9.4 Storage
6.9.5 Containers
6.9.6 Collection of Sharps
6.9.7 Mixed Waste
6.9.8 Waste Minimization
6.10 Nanomaterials
6.10.1 Engineering Controls
6.10.2 Work Practices
6.10.3 Personal protective Equipment
6.10.4 Training
6.10.5 Standard Operation Procedures
6.10.6 Consultation
7.0 BIOLOGICAL AGENTS
7.1 General
7.2 Responsibilities
7.2.1 General
7.2.2 Institutional Biosafety Committee Application
7.3 Containment Methods
7.3.1 Laboratory Practice
7.3.2 Safety Equipment (Primary Barriers)
7.3.2.1 Biological Safety Cabinets
7.3.2.2 Other Safety Equipment
7.3.3 Secondary Barriers
7.4 Biosafety Levels
7.4.1 Biosafety Level 1
7.4.1.1 Standard Microbiological Practices for BL1
7.4.1.2 Safety Equipment for BL1
7.4.1.3 Laboratory Facilities for BL1
7.4.2 Biosafety Level 2
7.4.2.1 Standard Microbiological Practices for BL2
7.4.2.2 Special Practices for BL2
7.4.2.3 Safety Equipment for BL2
7.4.2.4 Laboratory Facilities (Secondary Barriers) for BL2
7.4.3 Biosafety Level 3
7.4.4 Biosafety Level 4
7
7.5 Biological Spills
7.5.1 Sterilization, Disinfection, and Decontamination
7.5.2 Decontamination of Spills
7.5.3 Biological Spill in the Open Laboratory
7.5.4 Biological Spill within a Biological Safety Cabinet
7.5.5 Biological Spill in a Centrifuge or Other Equipment
7.5.6 Biological Spill on a Person
7.6 Human Blood, Blood Products, and Other Potentially Infectious Materials
7.6.1 Exposure Control Plan
7.6.2 Unit Specific Plan
7.6.3 HIV and HBV Research Laboratories and Production Facilities
7.6.4 Universal Precautions
7.6.5 Engineering and Work Practice Controls
7.6.6 Personal Protective Equipment
7.6.7 Housekeeping
7.6.8 Waste Disposal
7.6.9 Laundry
7.6.10 Hepatitis B Vaccination and Post exposure Evaluation and Follow-up
7.6.11 Communication of Hazard to Employees: Labels
7.6.12 Communication of Hazard to Employees: Information and Training
7.7 Recombinant DNA Activities
7.8 Animal Studies
7.9 Infectious Waste Management
7.9.1 Separation and Packaging of Infectious Waste
7.9.2 Storage and Transport of Infectious Waste
7.9.3 Infectious Waste Treatment
7.9.3.1 Steam Sterilization
7.9.3.2 Chemical Disinfection
7.9.3.3 Mixed Waste
8.0 RESEARCH INVOLVING ANIMALS
8.1 Physical Hazards
8.2 Allergens
8.3 Zoonoses
Appendix A FACT SHEETS
8
1. Emergency Response
2. Chemical Safety
3. Compressed Gas Cylinders
4. Highly reactive materials, High pressure Reactions, or Vacuum systems
5. Biological Safety
Appendix B WASTE MANAGEMENT LOGBOOK
Appendix C UNIT SPECIFIC PLAN FORM
STANDARD OPERATING PROCEDURES
9
LIST OF TABLES
Table 5.1 Properties of Protective Clothing Materials
Table 6.1 NIOSH Carcinogens
Table 6.2 Reproductive Toxins
Table 6.3 Quick Reference for Spill Cleanups
Table 6.4 Incompatible Materials: General Categories
Table 6.5 Incompatible Materials Chart
Table 6.6 Procedures for Inorganic Acid Neutralization (Does Not Apply to Chromic Acid)
Table 6.7 Flammable Liquid Classification
Table 6.8 Maximum Allowable Size of Flammable and Combustible Liquid Containers in
Laboratories
Table 6.9 Maximum Size and Quantity Limitations for Compressed or Liquefied Gas Cylinders in
Laboratories
Table 6.10 List of Peroxidizable Compounds
Table 6.11 Quick Guide: Exposure Risks and Control Measures for Common Laboratory Operations
Involving Nanomaterials
Table 7.1A Summary of Practical Disinfectants
Table 7.1B Summary of Practical Disinfectants (Use Parameters)
Table 7.2 Agent Summary Statements Available
Table 7.3 Arboviruses and Arenaviruses Assigned to Biosafety Level 2
Table 7.4 Vaccine Strains of BSL 3/4 Viruses Which May Be Handled at BLS2
Table 7.5 Arboviruses and Certain Other Viruses Assigned to Biosafety Level 3 (on the Basis of
Insufficient Experience)
Table 7.6 Arboviruses and Certain Other Viruses Assigned to Biosafety Level 3
1
0
LIST OF FIGURES
Figure 4.1 Medical Emergency Procedures
Figure 4.2 Medical Emergency Procedures, Students
Figure 6.1 General Manufacturer label
Figure 6.2 General Guidelines for Chemical Handling
Figure 6.3 Guidelines for Handling Acutely Toxic Chemicals
Figure 6.4 Guidelines for Working with Pesticides
Figure 6.5 Guidelines for Handling Chemicals with High Chronic Toxicity, Carcinogens, and
Reproductive Toxins
Figure 6.6 Procedures for Spills of Volatile, Toxic, or Flammable Materials
Figure 6.7 Procedures for Chemical Spill on a Person
Figure 6.8 Procedure for Cryogenic Liquid Spill on a Person
Figure 6.9 Procedure for Small, Low-Toxicity Chemical Spills
Figure 6.10 Mercury Spill Procedure
Figure 6.11 Examples of Chemicals Stored by Hazard

1
1
Acknowledgement
Penn State would like to thank Northwestern University for their help and generous donation of
the template for this document.
Plan developed in 2009
Revision Date Revision Summary
September 2012 Revised Plan to include updated Biosafety in
Microbiological and Biomedical Laboratories 2009
information
January 2013 Revised Plan to included Machine shop
information
April 2013 Added information regarding LRSP policy
July 2013 NIOSH Carcinogen table updated
Added information on Global Harmonization
System
October 2013 Updated Ansi shower flow requirements
June 2014 Added link to Fisher Scientific chemical storage
guidance
1
2

1
3
LABORATORY AND RESEARCH SAFETY PLAN
1.0 INTRODUCTION
Penn State University has established a policy for the management of laboratory and research hazards,
SY43Laboratory and Research Safety Plan policy,
http://guru.psu.edu/policies/SY43.html
The Laboratory and Research Safety Plan is made up of four sections, the General Safety Plan, Waste
Management Logbook, Rules and Procedures for the Use of Radioactive Material at The Pennsylvania State
University, and the Unit Specific section
The Laboratory and Research Safety Plan provides information and guidance to help you conduct your
laboratory and research work safely and in compliance with environmental health and safety regulations and
University policy. It is also a useful training resource for principal investigators and other supervisory
personnel.
The General Safety Plan serves as a reference source for a broad range of general safety issues for
laboratories and research areas. Your facility's Unit Specific Plan is the portion of the plan that addresses
hazards specific to your laboratory or research area. Users of Radioactive material should include a copy of
Rules and Procedures for the Use of Radioactive Material with these two sections and generators of
hazardous waste should include their Waste Management Logbook. These four sections should provide
comprehensive information to address hazards in your area. It is recommended that you also maintain
emergency plans; medical surveillance and scheduling information; and fit test reports and training
documents with your Laboratory and Research Safety Plan.
The Laboratory and Research Safety plan should be made available to all research and laboratory workers.
Although the information in this document is compiled from sources believed to be reliable, it is not all-
encompassing and is intended only to serve as a starting point for good safety practices. The laboratory or
research area manager or supervisor is responsible for adding specific information, for developing and
maintaining a safe workplace, and for complying with federal, state, and local laws and University policy.
Whenever used, the word shall indicates required procedures. The word should indicates a
recommendation for good practice.
The requirements for working with radioactive material can be found in SY-14 and the Rules and
Procedures for the Use of Radioactive Material. This Safety Policy and the Rules and Procedures require all
users of radioactive material to obtain prior authorization from the University Isotopes Committee before
acquiring any radioactive material. Contact EHS for details on how to apply for this authorization.
The requirements for working with Lasers can be found in SY-17. This Penn State Safety Policy has
specific registration, general training, laser specific training, and self inspection requirements. In addition,
the policy requires the issuance and required use of laser specific safety materials.

1
4
The requirements for working with radiation producing equipment (x-rays) can be found in SY-15. This
Penn State Safety Policy has specific registration, general training, and device specific training,
EMERGENCY NUMBERS
EHS 814-865-6391
FIRE, POLICE, AMBULANCE 911
UNIVERSITY POLICE, UP 814-863-1111
1
5

1
6
2.0 RESPONSIBILITIES
This section describes and assigns responsibilities associated with laboratory and research safety practices.
2.1 Penn State Leadership
The university president endorses the Penn State’s Environmental Health and Safety Policy SY01
requiring that the University leadership maintain a safe work environment within their jurisdiction, by
monitoring and exercising control over their assigned areas.
2.2 Senior Vice President for Finance and Business
Penn State University Safety policy SY01 includes the President’s statement to the entire PSU leadership of
his commitment to the University’s Environmental Health and Safety Policy. He has delegated
administrative responsibility for the laboratory and research safety programs to the Senior Vice President for
Finance and Business
2.3 Associate Vice President of Office of Physical Plant
The Associate Vice President of the Office of Physical Plant reports to the Senior Vice President for Finance
and Business and oversees the activities of EHS.
2.4 Environmental Health and Safety
The director of EHS reports to the Associate Vice President of Office of Physical Plant. EHS has overall
responsibility for the administration of the University’s environmental health and safety programs. Their
mission is to work with the campus community to develop and implement efficient, comprehensive and pro-
active health and safety programs.
EHS responsibilities include:
Developing safety programs that protect the health and safety of students, faculty, staff, visitors and
the environment.
Assisting the campus community in complying with federal, sate, and local regulations.
Providing oversight to help ensure conformance with these programs.
EHS representatives are authorized to enter University facilities within their jurisdiction at any time to
observe working conditions, monitor equipment, and sample for contaminants. EHS is authorized to close a
facility or stop a process or procedure that poses an imminent danger to life or property.
2.5 Budget Executives and Budget Administrators (Chancellors, Deans, Associate
Deans, Division Heads, etc.):
1
7
These functions have the primary responsibility to maintain a safe work environment within their
jurisdiction, by monitoring and exercising control over their assigned areas.
S/He must assign a representative from each academic and administrative unit to the University Safety
Council. This representative must be selected to ensure compliance with University safety policies, rules,
procedures and practices. This is often the individual designated to act on behalf of the budget executive
or budget administrator.
S/He must communicate to all faculty, employees and students that health and safety of persons in the
workplace and environment are of the highest priority at Penn State University.
S/He must ensure that health and safety responsibilities are carried out in the academic departments or
administrative units for which they are responsible.
S/He must ensure that environmental health and safety obligations established by this program applicable
to their areas of jurisdiction are carried out. This includes assuring compliance with applicable state and
federal health and safety rules, regulations, standards and procedures. Included, for example, are
regulations of the Pennsylvania Department of Environmental Protection (PADEP), and Nuclear
Regulatory Commission (NRC), and policies and procedures established by the Office of Environmental
Health and Safety.
S/He must monitor implementation of programs designed to protect the health and safety of faculty, staff,
students and visitors:
a. Consult with their University Safety Council representative and/or the Office of Environmental
Health and Safety with respect to new, existing or planned facilities or equipment that may
present a health or safety hazard to determine specific measures that may need to be implemented
to control these hazards before exposure to these hazards may occur.
b. Support measures such as training, use of protective devices, and resources to control and
prevent hazards.
2.6 University Safety Council
The University Safety Council is comprised of members representing academic colleges and
administrative units, as appointed by their respective budget executives. University Safety Council
representatives are commonly referred to as "Safety Officers."
The duties of the University Safety Council are to develop and implement, under the guidance of the
Office of Environmental Health and Safety, a comprehensive and practical occupational health and safety
program, and to maintain an environment that is conducive to the safety, health and well-being of the
University community.
Each member of the University Safety Council shall attend the regularly scheduled meetings and special
meetings of the University Safety Council, and report Council activities to the appropriate budget
executive.

1
8
S/He must establish and maintain, as chairperson, a Safety Committee within the member's area of
responsibility. The size and structure of this Committee shall be dictated by the types of activities, the
potential hazards inherent to those activities, and the number of persons who may be exposed.
S/He must accompany insurance company loss prevention representatives on inspections of areas under
the Safety Officer's jurisdiction.
S/He must review all Employer's Reports of Occupational Injury or Illness for employee accidents, or the
Incident Report for non-employees or employees not engaged in normal employment activities,
whichever report is appropriate for the accident/illness, and any other associated accident/illness reports.
S/He must assist in the investigation of all serious accidents, and all other accidents when requested by
the supervisor.
S/He must initiate proper follow-up measures and ensure corrective actions are implemented when unsafe
conditions, practices or equipment are reported or observed.
2.7 The Institutional Biosafety Committee
The Institutional Biosafety Committee (IBC) oversees the research and instruction involving biohazards.
Members are drawn from a variety of disciplines including chemistry, engineering, and biomedical sciences
and the community.
The committee's responsibilities do not include research involving ionizing and non-ionizing radiation.
Such activities are under the jurisdictions of the
University Isotopes Committee. Research involving
animals is under the jurisdiction of the Institutional Animal Care and Use Committee.
The IBC is responsible for reviewing and approving all research and instruction involving biohazards, as
defined in University Policy SY-24. Approval by the IBC must be granted prior to the use of any
biohazardous material.
2.8 Department Heads, Center Directors, and Other Facility Directors
The term department head will be used in this text to include center directors and other facility directors.
The Senior Vice President for Finance and Business and the Vice President for Research have assigned
direct responsibility for compliance with the University's safety and health programs to department heads.
This means that the department head shall provide a safe workplace and shall implement the safety and
health programs. This includes ensuring that personnel are adequately trained, and overseeing the
preparation and submission of annual laboratory safety self-audits. Department heads shall appoint building
safety officers and alternates.

1
9
The department head shall maintain discipline, enforce rules and regulations, and take prompt, effective
corrective action when necessary. The department head shall also provide assistance to EHS staff when
situations arise involving investigators and other personnel in the department.
The department head shall be familiar with and understand the federal, state, and local regulations and
University policies applicable to the department's work and shall ensure compliance through principal
investigators and other supervisory personnel. Regulatory and policy documents are available on the EHS
website, www.ehs.psu.edu and from EHS.
The department head may delegate safety and health-related tasks to principal investigators or other
supervisors, but ultimately compliance is the department head's responsibility
2.9 Principal Investigators and Supervisors
The PI or supervisor is responsible to the department head for the safe and legal conduct of research under
his or her purview. This responsibility shall not be delegated. The supervisor shall be aware of the physical
and health hazards associated with all materials present in his/her laboratory. In the event of an accident, the
principal investigator shall initiate appropriate emergency procedures.
All supervisors (department chairs, faculty, and other employees with direct oversight of University
activities and employees or students) have specific responsibilities to provide for the health and safety of
those supervised. They are in a key position in the organizational structure to carry out the department's
safety policies and to prevent injuries to their employees.
S/He must be thoroughly informed of appropriate University and Departmental safety policies, rules and
procedures and how they specifically apply to his/her responsibilities and authority including the LRSP.
S/He must inform all new and current employees and students that safety and health, and concern for the
environment, are priorities at Penn State and to inform them about safety and health policies, rules,
regulations and procedures, as well as their specific responsibilities (the next Section, below).
S/He must ensure that required safety equipment, devices and personal protective equipment and apparel
are provided and maintained, and are properly used by individuals working in their operations.
S/He must provide employees and students with instruction and assistance in the proper operation of
equipment or materials involved in any operation which may be potentially hazardous.
S/He must take prompt corrective action when unsafe conditions, practices or equipment are reported or
observed.
S/He must encourage prompt reporting of health and safety concerns.
S/He must promptly conduct a thorough investigation in all work-related injuries, illnesses and accidents,
submit appropriate recommendations on all accident reports, including the Employer's Reports of
Occupational Injury or Illness or the Incident Report , as appropriate, and follow through to ensure
corrective measures have been implemented.

2
0
S/He must coordinate or conduct inspections to maintain safe and healthful conditions, and address any
deficiencies that are identified.
S/He must provide for health and safety training.
S/He must provide financial support for health and safety improvements, or request assistance from the
next higher level of supervision regarding these requests.
The PI/supervisor shall prepare a Unit Specific Plan and shall make all laboratory personnel aware of the
plan. See Section 3.0 for information about the Unit Specific Plan. S/He shall be familiar with and
understand the rules, regulations, and University policies pertaining to the workplace. These encompass but
are not limited to the following items: training, record keeping, labeling of chemicals, labeling and proper
disposal of surplus and waste chemicals and biological materials, posting of warnings, medical surveillance,
inventory reporting, engineering controls, safe work practices, provision of personal protective clothing and
equipment, and access restrictions.
S/He ensures Standard Operating Procedures are developed for hazardous chemicals/operations
not covered in the LRSP.
S/He either acts as or assigns an individual in his/her laboratory the responsibility for overseeing
safety within the laboratory. This person serves as the Laboratory/Research Safety Officer.
S/He ensures employees, visitors, and students receive general Laboratory Safety training
provided by EHS.
S/He ensure employees, visitors and students receive unit specific training on hazards not
covered by general EHS safety training.
S/He ensures employees, visitors, and students read, comply and complete LRSP certification.
S/He notifies employees, visitors, or students of hazardous chemical monitoring results, if any.
S/He answers employee’s questions and concerns and forwards unresolved questions and
concerns to EHS for response...
S/He provides training and information on the LRSP including the Unit Specific Plan to all
affected employees, visitors, and students.
S/He reviews and updates Unit Specific Plan annually, ensuring completion of certification by all
affected lab/researchers.
S/He makes sure employees, visitors, or students who develop signs or symptoms associated
with hazardous chemical exposure are given an opportunity to receive medical attention. Makes
available to employees permissible exposure limits for hazardous chemicals, information on
signs and symptoms associated with exposures to hazardous chemicals used, and Safety Data
Sheets for hazardous chemicals used. EHS is available to provide guidance.
2
1
2.10 Laboratory/Research Safety Officer
Each laboratory/research Principle Investigator/Supervisor shall either act as, or assign, an individual in
his/her laboratory/research area the responsibility for overseeing safety within his/her area. This person
serves as the Laboratory/Research Safety Officer. In areas where chemicals are used, this person also
serves as the Chemical Hygiene Officer.
S/He monitors procurement, use, and disposal of chemicals used in the lab.
S/He ensures that appropriate self-inspections are maintained.
S/He provides input to PI’s/Supervisors on developing precautions and adequate facilities.
S/He knows the current legal requirements concerning regulated substances, as provided through
EHS resources.
S/He seeks ways to improve the chemical hygiene program.
2.11 The Individual
Each individual working in a laboratory or other work-site where hazardous materials are used shall know
and comply with the University's safety policies and rules and shall follow both oral and written instructions
from the principal investigator or supervisor. The individual shall report to the principal investigator any
unsafe conditions and any accident or exposure to chemicals or biological agents. If the individual receives
no response or an unsatisfactory response, s/he shall contact the department head or EHS.
All University employees and students have specific responsibilities to comply with established health
and safety policies, standards, rules, procedures and regulations. Compliance with these is essential to
create and maintain a healthy and safe environment at all University locations.
S/He must comply with applicable environmental health and safety policies, standards, rules, regulations
and procedures. These include safety-related signs, posters, warnings and written/oral directions when
performing tasks.
S/He must not perform any function or operation which is considered hazardous, or is known to be
hazardous without proper instructions and authorization.
S/He must only use equipment and materials approved or provided by the supervisor or instructor and for
which instruction has been provided by this or other experience.
S/He must become thoroughly knowledgeable about potential hazards associated with the work area;
knowing where information on these hazards is maintained and how to use this information when needed.
S/He must wear or use prescribed protective equipment.

2
2
S/He must report all unsafe conditions, practices, or equipment to the supervisor, instructor or safety
officer whenever deficiencies are observed.
S/He must inform the supervisor or instructor immediately of all work-related injuries or accidents and
obtain prompt medical attention when necessary.
S/He must provide information necessary for the supervisor or safety officer to adequately and thoroughly
complete the Employer's Report of Occupational Injury and Illness and any other associated
accident/illness reports.
2.12 Laws and Regulations
Numerous laws and regulations govern work with chemicals and biological materials and the
responsibilities of employers and employees. A list of the major regulatory acts follows.
FEDERAL LAWS
Occupational Safety and Health Act of 1970 (OSHA Act). Contains the general industry
regulations.
OSHA Hazard Communication Standard. Governs the use of hazardous chemicals in
nonlaboratory locations.
OSHA Occupational Exposure to Hazardous Chemicals in Laboratories Standard. Governs the use
of chemicals in laboratories. In general, the Laboratory Standard adopts the guidelines found in
Prudent Practices for Handling Hazardous Chemicals in Laboratories (published by the National
Research Council) and incorporates some elements of the Hazard Communication Standard.
OSHA Occupational Exposure to Bloodborne Pathogens Standard. Governs workplace exposure to
human blood, blood products, and other potentially infectious material in any occupational setting.
National Institutes of Health Guidelines for Research Involving Recombinant DNA Molecules.
EPA Superfund Amendments and Reauthorization Act (SARA), Title III: Emergency Preparedness
and Community Right-to-Know Act. Establishes responsibilities for chemical reporting to the
community.
EPA Worker Protection Standard (WPS). Protects farm workers from pesticide exposure.
EPA Clean Water Act. Provides for surface water protection and results in many environmental
regulations.
EPA Spill Prevention, Control, and Countermeasures Rule. Provides requirements for oil storage
and spill plans
Resource Conservation and Recovery Act of 1976. Governs hazardous waste disposal.
Other regulations of the U.S. Environmental Protection Agency.
Regulations of the U.S. Department of Transportation.
Regulations of the U.S. Department of Agriculture.
STATE LAW
2
3
OSHA Regulation Hazard Communication 1910.1200. Governs chemical safety in the workplace.
Pennsylvania Pesticide Control Act of 1973. Governs the licensing of applicators and the
application of pesticides
2.13 Students
Although the Pennsylvania laws apply only to employees (including student employees), it is the policy of
the Pennsylvania State University to ensure that all students who might be exposed to hazardous materials in
the course of their activities at the University are also adequately protected and trained. Students shall
receive instruction in the appropriate safety precautions for their specific teaching lab and will be expected
to follow the given rules.
2
4

2
5
3.0 THE LABORATORY AND RESEARCH SAFETY PLAN
3.1 Laboratory and Research Safety Plan
The Laboratory and Research Safety Plan is intended to be a central safety resource for the laboratory or
research area. The complete Safety Plan includes:
1. General Safety Plan
2. Waste Management Logbook
3. Rules and Procedures for the Use of Radioactive Material at The Pennsylvania State University
4. Unit Specific Plan
5. Recommended documents included with Plan:
Emergency Plans
Annual Reviews
Respirator Records: Medical Examinations and Fit Test Reports
Training Documentation
The Plan should be located in the laboratory or research area preferably in a holder on the back of the
room’s entrance door or to either side of the door inside the lab.
The department-level safety records, including accumulation area locations, self audits, training records and
a list of overseers, shall be maintained by the head of each department (or designee) where hazardous
materials are used.
3.2 Unit Specific Plan
The Unit Specific Plan is the laboratory-specific section of the Laboratory and Research Safety Plan
for research labs, teaching labs, research areas and common facilities (those shared by more than one
researcher). See Unit Specific Plan Form in Appendix C. In the case of shared facilities, the director,
coordinator, or designated facility supervisor for the center shall compile the Unit Specific Plan.
3.2.1 Initial Preparation
If chemical, biological or radioactive materials or reactive processes are used, the principal
investigator/supervisor shall prepare a Unit Specific Plan available for review by EHS. The Unit
Specific Plan contains the following sections; only sections that are appropriate need be completed.

2
6
I. Research Overview
II. Chemical Safety
III. Biological Safety
IV. Radiation Safety
V. Animal Related Hazards
VI. Physical Hazards
VII. Safety Precautions in Place
VIII. Certification Agreement
IX. Appendix
3.2.2 Change of Facility
A new Unit Specific Plan shall be prepared when new facilities are opened. The original Unit
Specific Plan will not be considered valid for the new space.
3.2.3 Addition of or Change to a Specific Project
A Unit Specific Plan shall be prepared or modified for new projects not covered by the original Unit
Specific Plan.
3.3 Inspections
The University has an inspection program for all laboratories and research areas. Self audits are conducted
annually by lab personnel. Laboratory inspections are also regularly conducted by the EHS staff.
Investigators may be asked to update the Unit Specific Plan and other information. The inspector may
examine general laboratory/research conditions, engineering controls, work practices, chemical storage, use
of personal protective clothing and equipment, signs and postings, and records. Workers may be
interviewed. Inspection findings are provided to the principal investigator/supervisor, department head and
safety officer.
2
7
GENERAL SAFETY PLAN
4.0 UNIVERSITY EMERGENCY INFORMATION
4.1 Where to Find Specific Information
This section provides general information about the University's emergency response programs.
For detailed emergency notification procedures and other general emergency information, including
fire safety, see the EHS website, www.ehs.psu.edu.
For emergency information relating to chemical spills and exposures, see Section 6.0.
For emergency information relating to biological spills and exposures, including exposure to
bloodborne pathogens, see Section 7.0.
Call for assistance when needed. Always call the University Police 911 if there is an explosion, fire, injury,
or spill-related evacuation. If there is a chemical or biological spill, call EHS, 814-865-6391 during
business hours and 863-1111 at University Park at other times.
4.2 University Emergency Response Plan
Police Services maintains the University's Emergency Response Plan and an Operation Plan for
emergencies. The Emergency Response Plan formalizes responses to all classes of emergencies, from small
events to catastrophes. In emergency situations, the role of University Police (UP) is to investigate the
situation, provide site security, implement the emergency plan, and establish communications. EHS will
advise and assist with hazardous-material spill control and cleanup. When the ability to respond adequately
to an emergency is beyond the capability of University personnel, UP will call the local fire department or
local hazardous materials response team.
4.3 Building Emergency and Evacuation Plans
In the event of a fire, hazardous material release, or other hazardous situation requiring emergency response
the first responder will:
Activate the fire alarm, if needed
Call 911
Evacuate the zone
Assist emergency personnel by providing information regarding
location of the incident, origin, and persons involved
4.4 Power Failure
2
8
In the event of a power failure when there is adequate emergency lighting, cap any open chemical containers
and close gas cylinders, perform an orderly shutdown of equipment and processes, and close the fume hood
sash. Leave immediately when the area has been secured and can be left unattended. Contact EHS or UP if
there is a possibility of an uncontrolled reaction in a process that cannot be shut down.
4.5 Incident (Accident) Reporting
All laboratory research incidents shall be reported to EHS, including spills, fires, or injuries. Laboratory
incidents shall be investigated. The supervisor shall be responsible for providing a written report of
investigation findings and corrective actions to EHS and ensuring that corrective actions to prevent repeat
incidents are undertaken.
EHS may also prepare an investigation report, as follow-up for the incident. Investigations are made and
reports written to learn the cause of the problem and what changes in procedures, equipment, or training
should be made to avoid other accidents.
4.6 EHS Assistance
EHS will respond to chemical spills. However, if the spilled material is not volatile and there is no
immediate fire or toxic hazard, cleanup may be done by area employees (under direction of the principal
investigator/supervisor or EHS). In situations involving a fire or toxic hazard, EHS will advise on
evacuation or other precautions to protect persons or property in the immediate area.
4.7 Personal Injury
4.7.1. Burn
If your clothing catches fire, decide very quickly how to put out the fire and minimize burns. The
following methods are in order of preference:
1. Get under a safety shower or other water source if one is immediately at hand.
2. If a safety shower is not immediately available, stop, drop, and roll to extinguish the fire,
holding your hands over your face to shield your face and eyes.
Assess the condition of the skin's burn area. If skin is not broken, run water over the burn area to
remove heat. If skin is broken, apply a dry, sterile dressing over the wound. Seek medical attention
as soon as possible.
4.7.2 Inhalation
Call 911 to solicit trained emergency medical personnel in the event of an emergency. A person
exposed to smoke or fumes shall be removed to uncontaminated air. Any victim overcome by
smoke or fumes shall be treated for shock. Give cardiopulmonary resuscitation (CPR) if necessary
2
9
and if trained personnel are available. If a person needs to be rescued from a contaminated area,
evaluate the possibility of harm to the rescuer before anyone enters or remains in the contaminated
area without proper protective equipment.
The SDS should accompany the victim to the medical treatment facility.
4.7.3 Ingestion
If a person ingests a toxic chemical, determine, if possible, what was ingested and notify the
emergency medical personnel by calling 911.
The SDS should accompany the victim to the medical treatment facility.
4.7.4 Puncture or Cut
When treating a victim with a puncture wound or cut, wear personal protective equipment (e.g.,
gloves) to minimize exposure to human blood, body fluids, or other chemical or biological
contamination. Apply a pressure pad or clean cloth firmly to the wound. Raise the wounded area
above the level of the heart to slow the bleeding. For severe bleeding or spurting, very firmly press
the pressure pad directly on the wound and apply pressure at the applicable body pressure point
above the wound to stop the flow of blood. In a severe injury, keep the victim warm, calm, and
oriented to prevent shock. Seek medical attention as soon as possible.
4.7.5 Needlestick
Needlesticks or other accidents involving skin punctures by a chemical or biological agent shall be
reported to the supervisor immediately and EHS as soon as possible. Appropriate medical testing,
treatment, and follow-up may be indicated and shall be provided as appropriate. When a
needlestick occurs, do not wait to report the incident and obtain medical attention. See Section
7.0 for more information on needlestick exposures to human blood and other potentially infectious
human materials.

3
0
FIG. 4.1 MEDICAL EMERGENCY PROCEDURES
In the event you are injured or exposed to a hazardous substance, follow these procedures to obtain medical
care and establish any Workers' Compensation benefits to which you may be entitled. All work-related
injuries and illnesses must be reported to your supervisor.
Medical services are provided to Penn State University employees at Occupational Medicine, (814) 863-
8492, and Mount Nittany Medical Center, (814) 234-6110.
PROCEDURES
I. Ambulatory victims (able to walk)
A. Inform your supervisor or designated departmental employee of your injury or illness.
B. Proceed to Occupational Medicine or Mount Nittany Medical Center to secure treatment.
II. Nonambulatory victims (unconscious or unable to walk)
A. Call 911.
B. Report the injury, victim's name, and location (building, floor).
C. Ask for an ambulance.
FIG. 4.2 MEDICAL EMERGENCY PROCEDURES, STUDENTS
Students in need of emergency medical assistance should call 911.
For other, less severe medical emergencies, call University Health Services, 863-0774 or local health care
panel provider.
Extreme caution should be taken when determining whether to transport an injured employee to medical
care in your personal vehicle. It is better to call for an ambulance if there is any chance complications could
arise during transport.
3
1

3
2
5.0 GENERAL SAFETY
Working safely requires having the proper containment equipment and engineering controls, wearing
appropriate personal protective equipment, using proper work practices, knowing safety information for the
materials and equipment used, and following safety instructions and protocols.
The general safety information in this section is provided to assist investigators and supervisors in planning
work and guiding those carrying out procedures.
Because each laboratory or research area is unique, judgment is required in interpreting general concepts for
individual settings. The Unit Specific Plan provides specific information for individual areas. If you have
questions or concerns about implementing general safety concepts or specific safety procedures, consult
your supervisor or EHS.
Some areas contain more than one type or category of hazardous material. For example, biochemistry
laboratories may work with chemicals, biological agents, and radioactive materials. In such cases, the
protective equipment and work practices to be used are those that provide protection against all the
hazardous agents.
The Laboratory Safety Self Inspection form provides a useful tool for evaluating you laboratory. This form
must be completed in January and a copy placed in your Waste Management Logbook. This same form can
be used for peer inspections of laboratories.
5.1 Personal Hygiene
Personal hygiene is extremely important to persons working in a laboratory. Contamination of food,
beverages, or smoking materials is a potential route of exposure to toxic chemicals or biological agents
through ingestion.
Laboratory personnel shall not prepare, store, or consume food or beverages; pipette by mouth;
smoke; apply lip balm or cosmetics; or handle contact lenses in the work area. This familiar
elementary safety rule shall be followed by everyone working in or visiting a laboratory.
Handwashing is a primary safeguard against inadvertent exposure to toxic chemicals or biological agents.
Always wash your hands before leaving the laboratory, even though you use gloves. Wash your hands after
removing soiled protective clothing, before leaving the laboratory, and before eating, drinking, smoking, or
using a rest room.
Wash your hands periodically during the day at intervals dictated by the nature of your work. Wash with
soap and running water, with hands held downward to flush the contamination off the hands. Turn the tap
off with a clean paper towel to prevent recontamination, and dry your hands with clean towels.
3
3
Confine long hair and loose clothing when in the laboratory to keep them from catching fire, dipping into
chemicals, or becoming entangled in moving machinery. Avoid wearing finger rings and wrist-watches
which may become contaminated, react with chemicals, or be caught in the moving parts of equipment.
Remove laboratory coats and gloves before you leave the laboratory to prevent spreading contamination
to other areas. Never wear contaminated or potentially contaminated gloves outside the laboratory. Change
gloves often and whenever contaminated.
5.2 Personal Protective Clothing and Equipment
Personal protective clothing and equipment protects you from injury due to absorbing, inhaling, or coming
into physical contact with hazardous materials. Some protection is afforded by ordinary clothing and
eyeglasses. You have a responsibility to dress sensibly for laboratory work. Laboratory clothing protects
workers' own clothing. You are responsible for using special protective clothing and equipment when they
are required for safety. Protective wear may include laboratory coats, wraparound gowns, cloth masks,
coveralls, aprons, gloves, shoe covers, and respirators. Select garments and fabric based on the nature of the
hazardous agent.
Personal protective clothing and equipment shall be used and maintained in a sanitary and reliable condition
and shall be cleaned regularly to avoid spreading contamination.
Laboratory coats shall never be washed at home. Regular clothing that is suspected of being contaminated
shall be evaluated by EHS for a proper decontamination or disposal method. It shall not be washed with or
come into contact with other personal laundry.
There are special precautions to be taken for clothing used during pesticide applications. Please see the
Penn State University Pesticide Management Program Manual for further instructions.
5.2.1 Clothing
Cover unprotected skin whenever possible. Suitable clothing shall be worn in the laboratory;
shorts are not appropriate. Clothing may absorb liquid spills that would otherwise come in contact
with your skin. Long sleeves protect arms and shall fit snugly, especially when you are working
around machinery. Wool affords more protection from flash burns or corrosive chemicals than
cotton or synthetic fabrics. Synthetic fabrics may increase the severity of injury in case of fire.
Cotton is less prone to static electricity buildup than nylon or other synthetics.
Wear substantial shoes in the laboratory or research area to protect against chemical splashes or
broken glass. Do not wear sandals, cloth sport shoes, perforated shoes, or open-toe shoes. If you
clean up a spill from the floor, you may need the added protection of rubber boots or plastic shoe
covers. Steel-toe shoes are required for handling heavy items, such as gas cylinders or heavy
equipment components.
Aprons, laboratory coats, gloves, and other protective clothing, preferably made of chemically
inert material shall be readily available and used. Laboratory coats are essential to protect
3
4
street clothing from biological agent aerosols or chemical splashes and spills, vapors, or dusts. For
work involving carcinogens, disposable coats may be preferred. For work with mineral acids, acid
resistant protective wear is desirable. See Table 5.1 for properties of protective clothing materials.

3
1
TABLE 5.1 PROPERTIES OF PROTECTIVE CLOTHING MATERIALS *
PROPERTIES
MATERIAL STRENGTH CHEMICAL
RESISTANCE
FLAMMABILITY STATIC
PROPERTIES
COMFORT USES
Cotton Fair durabilit
y
Degraded by acids;
binds
Special treatmen
t
for flame
N
o static problems Comfortable,
lightweight
Lab coats
Modacrylic Resistant to rips and
tears but less so than
polyamide fibers;
abrasion- resistant but
less so than nylon or
polyester
Resistant to most
chemicals
In direct flame, fabric
shrinks to resist flame
penetration; will not
melt or drip; self-
extinguishing; rapidly
dissipates when source
of ignition is removed
Has antistatic
properties
Comfortable, soft, and
resilient; easy to
clean; has soil release
properties
Lab coats
N
ylon Exceptionally strong
and abrasion resistant
N
ot water absorben
t
Melts when heated;
requires flame retardant
Static buildup
possible; requires
antistatic agent.
Lightweigh
t
Lab coats
Plastic Usually reinforced at
points of strain; will
not stick together,
peel, crack, or stiffen
Resistant to corrosive
chemicals
Can be ignited by
flammable solvents and
others in event of static
discharge
Accumulates
considerable charge
of static electricity
Lightweigh
t
Aprons, sleeve
protectors, boots
Polyolefin Resistant to rips and
tears
Excellent chemical
resistance; low
binding for chemicals
High melting point;
flame-resistant
Good static
dissociation
Lightweight; good
permeability; limited
moisture absorbency;
wearer perspiration
may cause discomfort
Bouffant caps
Polypropylene Strong Resistant to most
chemicals; oxygen
and light-sensitive
Low melting point;
requires flame retardant
Static buildup
requires antistatic
agent
Lightweigh
t
Aprons
Rayon Fairly durable Degraded by acids;
binds some
chemicals.
Lab coats
*Based on manufacturer's claims
From Chemical Safety Manual for Small Businesses, American Chemical Safety, second edition, 1992.

3
2
5.2.2 Eye Protection
Eye protection that meets ANSIZ87.1 requirements is mandatory in laboratories where there is
risk of flying objects, splashing chemicals, and corrosive vapors. Eyes are very vascular and can
quickly absorb many chemicals. Eye protection is not interchangeable among employees and shall
be provided for each individual unless disinfected after use.
Safety glasses with clear side shields are adequate protection for general laboratory use. Goggles
shall be worn when there is danger of splashing chemicals or flying particles, such as when
chemicals are poured or glassware is used under elevated or reduced pressure. A face shield (or
face shield with goggles) offers maximum protection (for example, with vacuum systems that may
implode). Face shields are designed to be used in conjunction with either safety glasses or goggles.
Corrective lenses in spectacles do not in themselves provide sufficient protection. Persons whose
vision requires corrective lenses, and who are required to wear eye protection, shall wear goggles
over their eyeglasses, prescription safety glasses, or goggles with prescription lenses.
5.2.3 Gloves
Gloves are worn to prevent contact with toxic or biological agents, burns from hot or extremely
cold surfaces or corrosives, or cuts from sharp objects. Skin contact is a source of exposure to
infectious agents and toxic chemicals, including carcinogens. Many gloves are made for specific
uses. For adequate protection, select the correct glove for the hazard in question.
A leather glove provides good protection for picking up broken glass, handling objects with sharp
edges, and inserting glass tubing into stoppers. However, because they absorb liquid, leather gloves
do not provide protection from chemicals, nor are they adequate for handling extremely hot
surfaces. Gloves designed to insulate against hot surfaces and dry ice are not suitable for handling
chemicals.
The chemical resistance of rubber or plastic gloves varies greatly according to the glove material
and the chemical handled. (Glove Selection Chart)
Chemicals can eventually permeate all glove materials. Select glove materials resistant to the
chemical being used, and change gloves periodically to minimize penetration. The chemical
resistance of common glove materials varies according to the glove manufacturer, as manufacturers
may vary the thicknesses and formulations of materials. Call the manufacturer to verify that a
particular glove material is suitable for the chemical in use.
Inspect gloves for punctures or tears before putting them on. To prevent contamination of your
hands or work surfaces, wash rubber or plastic gloves thoroughly with water before removing them.
Pull off disposable gloves inside out and dispose of them according to the contamination hazard.
Always remove contaminated gloves before leaving the laboratory. Always wash your hands after
handling chemicals or biological agents, before leaving the work area, and before eating, drinking,
smoking, or applying cosmetics.

3
3
5.2.4 Respirators
When feasible, engineering controls shall be provided to minimize airborne hazards. If
accepted engineering control measures are not available to prevent or protect against airborne
contaminants, employers are required to provide respirators at no cost to employees and employees
are required to wear them. EHS must evaluate area to determine hazard and appropriate controls.
Respirators are considered a last resort of protection against exposure to inhalation hazards after all
practicable engineering options have been exhausted.
Many kinds of respirators are available. The supervisor or principal investigator, after consultation
with EHS, is responsible for selecting an appropriate respirator for protection against a given
contaminant and for evaluating it in terms of the range of contaminants to which an employee is
exposed during a particular procedure.
An employee or student must meet certain qualifications before being allowed to wear a respirator.
The individual shall be examined by a licensed physician to determine whether he or she is in
sufficiently good health to wear the respirator. A medical history of respiratory or heart disease
could preclude the use of a respirator.
Annual fit testing is necessary to establish that the chosen respirator seals to the face properly to
prevent inward leakage of contaminants. Respirator wearers shall receive interactive training in
respirator use, limitations and care. The respirator shall be cleaned and disinfected on a regular
basis and inspected before and after each use.
Respirators shall not be worn when conditions prevent a good facepiece-to-face seal, as with beard
growth, sideburns, or dentures. With full-face respirators, temple bars on eyeglasses interfere with
the sealing edge of the facepiece.
Persons desiring to use a respirator shall inform EHS and obtain information on the requirements.
The formal written respiratory protection program for the University is available on the EHS
website,
www.ehs.psu.edu and respirator wearers must read this document in its entirety.
5.3 General Laboratory Protocol
All laboratory protocols shall include basic safety precautions. These include personal hygiene, work
practices, and the appropriate personal protective clothing and equipment needed to protect you from
exposure to chemicals or biological agents. Each situation is unique, and safety aspects shall be assessed
individually as described in your laboratory's Unit Specific Plan. Some of the fundamental principles of
laboratory operation are described below.
5.3.1 Housekeeping
In the laboratory and elsewhere, keeping things clean and organized helps provide a safer
environment. Keep drawers and cabinet doors closed and electrical cords off the floor to avoid
tripping hazards. Keep aisles clear of obstacles such as boxes, chemical containers, and other
storage items that might be put there even temporarily. Avoid slipping hazards by cleaning up
spilled liquids promptly and keeping the floor free of stirring rods, glass beads, stoppers, and other
3
4
such hazards. Never block or even partially block the path to an exit or to safety equipment, such as
a fire extinguisher or safety shower.
Make sure that supplies and equipment on shelves provide sufficient clearance so that fire sprinkler
heads operate correctly. Sprinkler heads require at least 18 inches of vertical clearance.
Broken glass and other sharp items shall be disposed of in rigid, puncture-resistant containers to
protect persons collecting the waste materials; maroon barrels for broken glass disposal are located
in most locations. Needles and syringes that are not contaminated may be sealed in a rigid,
puncture-resistant container and placed in infectious waste barrels for disposal. When discarding
empty boxes or other containers bearing hazardous materials labels, the labels shall be defaced or
removed before disposal. Contaminated boxes or containers shall not be disposed of in the regular
trash.
Chemical wastes and unwanted chemicals shall be disposed of promptly and not left to clutter a
laboratory. The procedure is described in Section 6.0. Infectious waste management is described in
Section 7.0. Additional information on disposal of human body fluids or other potentially
infectious materials appears in Section 7.0.
5.3.2 Cleaning Glassware
When cleaning laboratory glassware, wear appropriate gloves that have been checked for tears or
holes. Avoid accumulating too many articles in the cleanup area around the sink; space is usually
limited, and piling up glassware leads to breakage. Do not clean food containers in a sink that is
used for cleaning contaminated glassware.
Many fingers have been badly cut by broken glass from glassware that was intact when put into the
water. Handle glassware carefully, and watch out for broken glass at the bottom of the sink. A
rubber or plastic mat in the sink will help minimize breakage.
Avoid using strong cleaning agents such as nitric acid, chromic acid, sulfuric acid, strong oxidizers,
or any chemical with "per" in its name (perchloric acid, ammonium persulfate, etc.). The prefix
"per" signifies a state of completeness or extremity. In a chemical name, it denotes 1) a compound
containing an element in its highest state of oxidation, such as perchloric acid; 2) the presence of
the peroxy group (-O-O-), as in peracetic acid; or 3) exhaustive substitution or addition, as in
perchloroethylene.
If you must use these substances for cleaning, you should be thoroughly familiar with their
hazardous characteristics and use appropriate protective equipment. Flammable solvents such as
acetone should be used in minimum quantities for cleaning and with appropriate precautions taken
during their use. Acids and solvents, except those covered by the Drain Disposal Guideline, shall
not be rinsed down the drain during cleaning but shall be collected for proper treatment and
disposal.

3
5
5.3.3 Laboratory Animals
Federal regulations require that the Animal Care and Use Committee review and approve the use of
animals in research.
Laboratory animals may be potential sources of hazardous chemical exposure from metabolic
products, wastes, cage litter, and contaminated cages. The preparation of food and water containing
toxic substances under investigation shall be done with all precautions ordinarily taken to protect
the health and safety of personnel. Precautions in administration of toxic substances, aerosol
suppression, personal protection, and waste disposal shall be taken.
5.3.4 Relocating or Closing a Laboratory
Guidelines are available from EHS website, www.ehs.psu.edu, to assist you in safely relocating
laboratory chemicals or biological agents within the University.
All chemicals that will not be relocated shall be listed on a chemical pick-up request form available
on the EHS website, www.ehs.psu.edu. The form shall be completed before the principal
investigator relinquishes possession of the vacated laboratory. Disposition of all unwanted
chemicals is the responsibility of the principal investigator. The department of record is responsible
for the safe and lawful cleanup and disposition of all chemicals and biological materials that are
abandoned. All biological materials shall be autoclaved or chemically disinfected and disposed of
before the laboratory is vacated.
Surfaces and equipment potentially contaminated with hazardous chemicals or biological agents
shall be decontaminated before the laboratory is vacated. The principal investigator/supervisor is
responsible for ensuring that the equipment is properly decontaminated. Accessible surfaces
(chemical fume hoods, sinks, benchtops) should be cleaned, when practical, by the principal
investigator and staff. If this is not possible, an outside contractor specializing in the industrial
testing and cleaning of contaminated laboratory equipment should be contacted. The principal
investigator shall provide the contractor with thorough and accurate information pertaining to the
past uses of the equipment.
5.3.5 Transportation of Hazardous Materials
The U.S. Department of Transportation (DOT) requires that a licensed hazardous materials
transporter be employed if hazardous materials are transported on a public highway or by air or
water. DOT also requires that all individuals offering a hazardous material for transport receive
training. The material to be shipped shall be properly packaged in accordance with all applicable
regulations, and appropriate shipping papers shall be provided, see Hazardous Material Shipping
Guidelines.
A personal vehicle shall not be used to transport hazardous chemicals. Biological materials shall be
shipped in compliance with DOT and Centers for Disease Control and Prevention regulations.
Transport of regulated plant or animal pathogens shall comply with U.S. Department of Agriculture
regulations.

3
6
5.3.6 Laboratory Doors
Fire and life safety codes as well as University policy require that laboratory doors be kept closed at
all times. Keeping doors closed also helps ensure that ventilation systems work properly. This is
especially important in newer buildings with energy conservation systems.
5.3.7
Children in Laboratories
Laboratory areas contain many physical, chemical, biological or other potential health and safety
hazards. Children are likely to have a limited understanding of these hazards, and should be kept
away from areas where known hazardous conditions exist. Penn State University prohibits
children under the age of 16 from entering areas where known or suspected laboratory hazards
exist.
High school students that wish to work in Penn State laboratories must provide parental consent
and insurance documentation to the University on the paperwork available here.
5.4 General Laboratory Techniques
5.4.1 Laboratory Ventilation
Laboratories shall be provided with general ventilation adequate for employee comfort and
sufficient to supply air for hoods and other local ventilation devices. Because the general air supply
is not adequate for manipulating hazardous materials in the open, volatile or toxic chemicals shall
be handled in a chemical fume hood or other appropriate containment device.
Laboratory ventilation shall change the air at least six times per hour, depending on the nature of the
laboratory work. Except in special circumstances approved by EHS, air in laboratories shall be at a
negative pressure with respect to the rest of the building. Air diffusers or grilles shall be so
designed and located as to direct the air over the laboratory personnel and sweep the contaminated
air away from their breathing zone. To promote uniform distribution and mixing of air in large
laboratories, the supply registers shall deliver the air in all directions, at a typical velocity of 20
linear feet per minute.
Problems with general ventilation shall be reported promptly to the Office of Physical Plant (OPP).
Adjustments or alterations to the general ventilation equipment of a laboratory shall be performed
only under the supervision of OPP.
On occasion, OPP will issue notices of intent to perform maintenance work on the ventilation
system. These notices shall be heeded and chemical fume hoods shall not be used when OPP is
involved in repairing or adjusting the ventilation system. In the event that a hood in a particular
laboratory is being repaired, the supervisor of the laboratory is responsible for ensuring that the
OPP crew is informed of the hazards in the area. The hood shall be cleared of toxic materials and
properly decontaminated before the work begins. A Laboratory/Equipment Safety Clearance Form
must be completed and submitted to EHS.
3
7
5.4.2 Chemical Fume Hoods
A chemical fume hood is an important engineering control for preventing exposure to hazardous
materials. In conjunction with sound laboratory techniques, a chemical fume hood serves as an
effective means for capturing toxic, carcinogenic, offensive, or flammable vapors or other airborne
contaminants that would otherwise enter the general laboratory atmosphere. With the sash lowered,
the fume hood also forms a physical barrier to protect workers from hazards such as chemical
splashes or sprays, fires, and minor explosions. Fume hoods may also provide effective
containment for accidental spills of chemicals, although this is not their primary purpose.
Additions, such as shelving; tampering with or changing damper settings; or any other alterations to
the chemical fume hood structure may reduce their performance and is prohibited.
Hoods are not meant for storage of chemicals. Volatile and odorous chemicals and highly toxic
gases shall be stored in ventilated cabinets. Storing chemical containers and equipment in a hood
impairs the performance of the hood. Hoods are backup safety devices to protect against toxic
vapors or dusts if an accident occurs or if the design of an experiment fails. The deliberate release
and venting of chemicals (i.e., evaporation) in hoods shall never be used as a means of disposal.
Apparatus in hoods shall be fitted with traps, condensers, or scrubbers to remove toxic fumes, gases,
vapors, or dusts before venting to the atmosphere. Hood performance is dependent on the room's
air flow pattern, including air flow generated by drafts and persons walking by. Equipment in the
hood should be stationed at least six inches away from the sash. This practice can reduce vapor
concentrations at the hood face by about 90 percent. Sashes should be pulled down as far as
workable when in use.
Hoods used for hazardous chemicals shall have an average face velocity of 80 - 100 feet per minute.
Compounds such as perchloric acid or aqua regia are likely to cause hood corrosion and only hoods
designed for perchloric acid use may be used. Please refer to Section 6.0 for further information.
Hoods shall be evaluated for performance regularly by EHS. The fans and duct systems are
maintained and inspected by OPP. Any problems with hood ventilation or airflow should be
reported to EHS or OPP for inspection and evaluation.
5.4.3 Safety Showers
Safety showers shall be installed in all areas where employees may be exposed to splashes or spills
of materials that may be injurious to the eyes and body. As a general rule, new shower installations
shall adhere to the recommendations for shower location and minimum performance requirements
established in American National Standard Z-358.1. Showers shall be placed as close to the hazard
as possible, but in no case more than 10 seconds travel time from the hazard. Department heads
shall ensure that safety showers are installed in the department where needed.
Every laboratory employee shall be instructed in the location(s) and use of a safety shower. Ideally,
a person should be able to find the shower with his or her eyes closed, if the shower is within
reasonable distance. Safety showers shall provide a minimum of 20 gallons of water per minute
and deliver the volume at low velocity; a high-velocity shower could further damage injured tissue.

3
8
Ideally, the water temperature of the shower should be between 60
o
and 95
o
F to prevent pain or
shock to a person standing under it for 10 to 15 minutes. Safety showers shall have quick-opening
valves requiring manual closing so that a person does not have to hold the valve open while trying
to undress or wash off. The pull handle shall be a delta bar or large ring within easy reach but not
so low as to be in the way.
Access to the shower or the activating handle shall not be impeded. The floor shall be clear in a 36-
inch-diameter area under the shower.
Showers shall be evaluated for performance regularly by EHS. The plumbing is maintained and
inspected by OPP. Any problems with water flow or shower operation should be reported to EHS
or OPP for inspection and evaluation.
5.4.4 Eyewash
New eyewash installations shall adhere to the recommendations for minimum performance
requirements established in American National Standard Z-358.1. Eyewash fountains shall supply
0.4 gallons of water per minute. The two basic kinds of eyewash fountains are the fixed-base
shower, much like a drinking fountain, with arm or foot-pedal operation, and the handheld-hose
type, with an aerating nozzle and lever-operated valve.
The fixed-base type has the advantage of multistream, cross-flow washing that can flush the face
and eyes at the same time. Faucet-mounted cross-flow eyewash fountains are also available. The
hose type has the advantage of also serving as a minishower for splashes on the arms, hands, and
other limited areas of the body. Contact EHS for information on the types of eyewashes available.
In older laboratories where eyewashes were not originally provided, faucet-mounted models may be
appropriate.
Gravity-feed eyewash devices (wall-mounted or on mobile carts) are not recommended unless they
provide adequate water supply for 15 minutes of eye washing and the stored water is treated so that
it does not become microbially contaminated. For such units, a documented monthly maintenance
program shall be established to ensure that the water supply remains in satisfactory and usable
condition. Bottle-type portable eyewashes are not acceptable, as they do not have the capacity to
deliver 0.4 gallons of water per minute.
Principal investigators are responsible for ensuring that eyewash fountains are tested weekly to
ensure that the valves operate properly, the required volume and aerated stream are available, and
the pipes or hose are cleared of sediment that might collect. A form for recording testing
information is available on the EHS website, www.ehs.psu.edu
Eyewash fountains shall be evaluated for performance regularly by EHS. The plumbing is
maintained and inspected by OPP. Any problems with water flow or operation should be reported
to EHS or OPP for inspection and evaluation
5.4.5
Pesticide Decontamination Supplies
3
9
Decontamination supplies are required to be located at all pesticide mixing and loading sites, at
areas within ¼ mile of areas where pesticides are being applied, and at areas where applicators
remove their PPE.
At the required locations, there must be sufficient water to wash the entire body in the case of an
emergency, which shall be at a minimum, three gallons for each pesticide applicator that may be
mixing or loading at any one time. A clean hose with running water meets this requirement. In
addition, soap and single-use towels must be supplied in sufficient quantities to meet the needs of
all of these applicators. There must also be clean clothes, such as one size fits all coveralls in
case the pesticide applicator needs to change.
Eyeflush is also required at all pesticide mixing and loading sites and at areas within ¼ mile of
areas where pesticides are being applied. However, if a pesticide label requires eye protection,
the eyeflush must be immediately available. This has been defined to mean within 10 seconds
reach. In order to meet this requirement for outdoor applications, a sealed pint bottle of water or
saline solution is allowed. This type of eyeflush should be discarded if partially used, out of date,
or if the seal has been broken. At other locations such as greenhouses and laboratories an
emergency eyewash station or running water meets this requirement
.
5.4.6 Laboratory Sinks and Drain Traps
Every laboratory using chemical or biological agents shall have at least one sink, preferably located
near the room exit, available for handwashing. The sink shall be cleaned regularly to eliminate
contamination, and soap shall be supplied for handwashing.
Drain traps in sinks, floors, and other places will dry out if they are not used regularly, allowing
odors and contamination to back up into the room. Drain traps shall be kept filled with water to
prevent backup. Also fill cup sinks on benches and in hoods.
5.4.7 Electrical Equipment
Electrical currents of very low amperage and voltage may result in fatal shock under certain
circumstances. Voltages as low as 24 volts AC can be dangerous and present a lethal threat. Low-
voltage DC circuits do not normally present a hazard to human life, although severe burns are
possible. The duration of contact with a live circuit affects the degree of damage, especially with
regard to burns.
All electrical switches shall be labeled, including circuit breakers in the service panels, and all
laboratory personnel shall know where these controls are and how to shut off circuits or equipment
in case of fire or other accident. Any electrical equipment that is not operating properly or seems to
be overheating shall be turned off immediately and inspected by a qualified technician.
Electrical equipment should be inspected periodically to confirm that the cords and plugs are in safe
condition. Circuit diagrams, operating instructions, descriptions of hazards, and safety devices are
usually provided by the manufacturer and should be kept on file for reference.
4
0
Only three-wire grounded, double insulated, or isolated wiring and equipment shall be used in
110V-115V AC applications. All wiring and equipment shall comply with the National Electrical
Code.
Electrical extension cords should be avoided where practical by installing additional electrical
outlets. When they are used, the wire gauge shall be equal to or larger than the size of the cord
being plugged into them. Electrical cords on equipment shall be discarded or repaired if frayed or
damaged. Cords should be kept as short as practical to avoid tripping hazards and tangles.
Place electrical equipment so as to minimize the possibility that water or chemicals could spill on it
or that water could condense and enter the motor or controls. In particular, place such equipment
away from safety showers. In cold rooms, minimize condensation by mounting electrical
equipment on walls or vertical panels.
Electrical equipment shall be de-energized and tagged or locked out according to PSU Electrical
Safety Program requirements before repairs are made. If adjustments or other contact are to be
made with energized electrical equipment, a second person shall be present. Be sure you are not on
a damp surface or touching a potential grounding surface. Use insulated tools, wear appropriate
flash resistant clothing, keep your hands dry, and wear safety glasses to prevent injury from sparks.
If a worker receives an electrical shock and is in contact with the energized device, use non-
conducting gloves or a non-conducting device to pull or push the victim free from the current
source. Help victims only if you are certain that you will not endanger your own safety. Turn off
the current if possible. Call 911. If a trained person is available, start CPR if necessary. Get medical
assistance at once.
5.4.8 Static Electricity
Static electricity may be generated whenever two surfaces are in contact with one another.
Examples are processes such as evaporation, agitation, pumping, pouring of liquids, or grinding of
solids or powders. Equipment used in these operations shall be bonded and grounded to prevent
static charges from accumulating on the containers. Blanketing with inert gas may also prevent
sparks in equipment where flammable vapors are present. Static electricity is increased by low
absolute humidity, as is likely in cold weather. Some common potential sources of electrostatic
discharges are ungrounded metal tanks and containers; metal-based clamps, nipples, or wire used
with non-conducting hoses; high-pressure gas cylinders upon discharge; and clothing or containers
made of plastic or synthetic materials.
5.4.9 Centrifuges
If a tabletop centrifuge is used, make certain that it is securely anchored in a location where its
vibration will not cause bottles or equipment to fall. Centrifuge rotors shall be balanced each time
they are used. Securely anchor and shield each unit against flying rotors. Regularly clean rotors
and buckets with non-corrosive cleaning solutions.
Always close the centrifuge lid during operation, and do not leave the centrifuge until full operating
speed is attained and the machine appears to be running safely without vibration. Stop the
4
1
centrifuge immediately and check the load balances if vibration occurs. Check swing-out buckets
for clearance and support.
5.4.10 Vacuum Pumps
If vacuum pumps are used with volatile substances, the input line to the pump shall be fitted with a
cold trap to minimize the amount of volatiles that enter the pump and dissolve in the pump oil. The
exhaust from evacuation of volatile, toxic, or corrosive materials shall be vented to an air exhaust
system. A scrubber or trap may also be required.
If pump oil becomes contaminated with toxic chemicals, it will exhaust the chemicals into the room
air during future use. Pump oil shall be changed if it becomes contaminated. Dispose of used pump
oil with EHS.
Before using the vacuum pump, ensure that the moving parts have been properly guarded and that
there are no exposed points of operation (i.e., exposed belt) that could nip a finger or catch hair or
clothing. Wear eye protection when working with a vacuum pump or setting up the cold trap
assembly.
5.4.11 Drying Ovens and Furnaces
Volatile organics shall not be dried in ovens that vent to the room air. Glassware rinsed with
organics should not be oven dried unless it is first re-rinsed with water. Non-mercury thermometers
rather than mercury thermometers shall be used for measuring oven temperatures. Nothing should
be stored on top of ovens. Careful attention should be used when using ovens to dry plastic pipette
tips to ensure the temperature is properly maintained.
Wear heat-resistant gloves and appropriate eye protection when working at ovens or furnaces.
ANSI-approved eyewear (i.e., heat-absorbing, reflective goggles) offers protection against
projectiles and infrared radiation.
5.4.12 Syringes and Scalpel Blades
Syringes used with hazardous agents shall have needle-locking or equivalent tips to assure that the
needles cannot separate during use. Disposal of needles and syringes contaminated with infectious
agents is described in Section 7.0. Do not recap needles after use. Recapping of needles
potentially contaminated with human blood, blood products, or other potentially infectious
materials is prohibited.
Syringes, needles, or scalpels shall be disposed of immediately after use in sealable, puncture-
resistant, disposable containers that are leakproof on the sides and bottom. The containers shall be
appropriately labeled as to the chemical or biological hazard. These sharps containers shall be
easily accessible to personnel in the immediate area of use.
5.4.13 Facility Cleaning and Maintenance

4
2
OPP janitors wet-mop floors (including laboratory space) on a weekly basis. However, building
services and custodial staff are prohibited from cleaning up chemical and biological materials
(including spills), and custodians shall not be expected to mop any floors that have not been
properly decontaminated after a spill.
In preparation for the cleaning service, the laboratory staff shall remove hazards that the
housekeeping workers might encounter during their activities. Chemical containers on the floor and
all containers of biohazardous waste shall be moved by laboratory occupants to a safe and secure
location before custodians enter the lab. In the event that a supervisor does not wish a particular
laboratory to be disturbed, custodial floor cleaning can be suspended on request of the area
occupants. To have the mopping discontinued, contact Area Services and post a sign on the lab.
Likewise, if maintenance is required on any component of the laboratory, such as a sink or piece of
equipment, the same principles of preparation apply. The supervisor shall again ensure that the
immediate area is decontaminated and any infectious agents or chemicals removed to another
secure area prior to initiation of work. Further, the laboratory supervisor shall inform maintenance
personnel of the presence of any hazardous materials to which they might become exposed. A
Laboratory/Equipment Safety Clearance Form, available on the EHS website, www.ehs.psu.edu,
must be completed, taped to the area and a copy sent to EHS.
Cleaning duties that are the specific responsibility of laboratory personnel shall be conducted on a
regular basis to prevent accidental contact with hazards and to reduce clutter in the lab space.
Laboratory equipment, including refrigerators, freezers, and work surfaces, shall be cleaned by
laboratory staff. In laboratories using large amounts of powdered carcinogens, reproductive toxins,
or acutely toxic materials, lab workers should avoid dry mopping or sweeping with a broom if this
could cause the materials to become airborne.
Facility maintenance and custodial staff shall not handle or remove hazardous waste bags or other
hazardous containers.
5.4.14 Glassware
Borosilicate glassware, such as Pyrex 7740, is the type preferred for laboratory experimentation,
except in special experiments involving ultraviolet or other light sources or hydrofluoric acid, for
which polypropylene containers are most appropriate. Measuring glassware, stirring rods, tubing,
and reagent bottles may be ordinary soft glass. Vacuum or suction flasks shall be designed with
heavy walls. Dewar flasks and large vacuum vessels shall be taped or otherwise screened or
contained in metal to prevent glass from flying if they should implode. An ordinary thin-walled
thermos bottle is not an acceptable replacement for a Dewar flask.
Because it can be damaged in shipping, handling, or storage, inspect glassware carefully before
using it to be sure it does not have hairline cracks or chips. Even the smallest flaw renders
glassware unacceptable and possibly dangerous. Flawed glassware shall be discarded in a rigid,
puncture-resistant broken-glass maroon container. Where the integrity of glassware is especially
important, it can be examined in polarized light for strains.
5.4.15 Assembling Apparatus

4
3
Operations that may generate airborne contaminants or that use flammable liquids or toxic, reactive,
or odoriferous materials shall be conducted in a chemical fume hood or other appropriate
containment enclosure. Whenever hazardous gases or fumes are likely to evolve, an appropriate
trap, condenser, or scrubber shall be used to minimize release of material to the environment.
Apparatus should be set up well back from the edge of the work area, be it a bench or a hood.
When assembled in a hood, apparatus should not obstruct the area. To avoid overflow, choose
apparatus with at least 20 percent more capacity than would normally accommodate the volume of
chemical planned for the operation. All parts of the apparatus shall be firmly balanced and
supported. Tubing shall be fastened with wire or appropriate clamps.
Stirrer motors and vessels shall be positioned and secured to ensure proper alignment. Magnetic
stirring is preferable, and non-sparking motors or air motors shall be used in any laboratory that
might contain flammable vapors.
Funnels and other apparatus with stopcocks shall be firmly supported and oriented so that gravity
will not loosen the stopcock plug. Use a retainer on the stopcock plug, and lubricate glass
stopcocks. Do not lubricate Teflon stopcocks.
Include a vent in apparatus for chemicals that are to be heated, and place boiling stones in unstirred
vessels. If a burner is to be used, distribute the heat with a ceramic-centered wire gauze. Insert a
thermometer in heated liquids if dangerous exothermic decomposition is possible. This will provide
a warning and may allow time to remove the heat and apply external cooling.
A pan under a reaction vessel or container will confine spilled liquids in the event of glass breakage.
If a hot plate is used, be sure that its temperature is less than the autoignition temperature of the
chemicals likely to be released and that the temperature control device does not spark. Whenever
possible, use controlled electrical heaters or steam in place of gas or alcohol burners.
5.4.16 Fire Extinguisher Policy
Fire extinguishers are provided by the University in corridors, public areas, and other locations as
required by building and life safety codes. It is the responsibility of the principal investigator or the
department head to ensure that appropriate fire extinguishers are purchased and installed in
laboratories. Contact the Fire Safety Engineer (EHS) for assistance in selecting fire extinguishers.
Fire extinguishers may be purchased through OPP. OPP will inspect and maintain all fire
extinguishers, both inside and outside laboratories. When fire extinguishers are installed or moved
to a new location, OPP must be contacted.
Basic fire prevention and fire safety training; and fire extinguisher training is provided by EHS.
5.4.17 Special Precautions against Ultraviolet Light
Germicidal lamps using ultraviolet light are common fixtures in biological safety cabinets, where
they serve to destroy bacteria and molds. These lamps are considered a high-level source of UV

4
4
radiation; exposure to the lamps without adequate personal protection could result in skin or eye
injury.
Acute skin effects due to direct UV exposure vary with dose. Dermal effects include three types:
erythema (sunburn), increase in pigmentation (sun tanning), and hyperplasia (increase in epidermal
cell growth, resulting in enlargement of tissue). UV radiation may also increase the cutaneous
effects of certain solvents and photosensitizing chemicals.
Eye injuries attributable to UV exposure are most prevalent among welders. Laboratory
applications are unlikely to achieve doses comparable to those in industrial settings, but a small
amount of UV light may produce temporary eye injury, such as corneal inflammation and "sand-in-
the-eye" sensation.
A great concern with UV eye exposure is that the victim is often unaware that damage is occurring.
Usually, no pain develops from the eye injury until four to six hours after the exposure. The only
way to prevent injury is to minimize eye exposure to UV light.
Appropriate protection against UV exposure includes long sleeves and laboratory gloves. For
individuals particularly sensitive to UV light, suntan lotion on the exposed skin of the face is
recommended. ANSI-approved shaded eye protection with side enclosures shall be worn in the
vicinity of a UV light fixture not shielded by a physical barrier.
5.5 Signs and Labels for Laboratories
Various signs and labels are required by government regulations and University policy. The
principal investigator shall obtain and post the signs and labels required for the laboratory. Most
required signs and labels may be obtained from EHS. The following signs and labels are required
for all laboratories in University facilities:
A "Laboratory Information" sign shall be posted outside all laboratories on the outside of
the door. Electronic copies are available on the EHS website, www.ehs.psu.edu ; hard
copies on sticker backing are available by request through EHS. This sign includes blank
areas to be filled out by laboratory personnel on information on specific hazards in the
laboratory, telephone numbers of responsible faculty and staff and emergency contacts.
The information provided on these signs shall be updated as necessary.
An "Emergency and laboratory Safety phone numbers” sign shall be posted in a prominent
location inside the laboratory, near the door or telephone. Electronic copies are available
on the EHS website, www.ehs.psu.edu. This sign lists who to call and their telephone
numbers in the event of an emergency.
A label bearing the University Police emergency number shall be placed on each telephone
in the laboratory.
5.6 Training

4
5
Training is required under the Hazard Communication Standard, for Chemical and chemical Waste
handling, Bloodborne Pathogens, and various general industry standards such as the Respiratory
Protection Program. University policy prohibits persons without appropriate training from working
in laboratories and other areas where hazardous chemicals are used. Federal law mandates
training at the time of initial assignment to a laboratory or work area where hazardous
chemicals are present or exposure to bloodborne pathogens is possible. Additional training is
required whenever a new chemical exposure hazard is introduced. Refresher training shall be
conducted annually for persons working in areas of potential exposure to bloodborne pathogens
and for persons with potential exposure to chemical hazards.
Principal investigators shall ensure that laboratory personnel are properly trained and shall certify
training on the Unit Specific Plan. Bloodborne-pathogens training information may be found in
Section 7.0.
A training table listing required and recommended training is available on the EHS website at
http://www.ehs.psu.edu/training/training_matrix%5Cindex.html In addition, EHS can provide
general safety seminars for laboratory or department groups. EHS training is general in nature;
principal investigators are required to provide specific safety training in the particular hazards of
their laboratories.
5.7
Machine Shop Equipment
PSU has a machine shop safety program that is intended to prevent injuries which may occur in a
shop environment. The program is oriented towards work in student and employee shops, but
many of the requirements also apply to work performed using shop equipment in labs that fall
under the scope of the PSU Laboratory & Research Safety Plan.
Shop equipment includes, but is not limited to, items such as belt sanders, miter saws, band saws,
drill presses, lathes, milling machines, radial arm saws, table saws and routers. Labs with this
type of equipment must incorporate the necessary requirements contained in the PSU Machine
Shop Safety Program. The specific requirements that must be implemented and complied with
include General Shop Safety Training, Equipment Specific Training, Monitoring, Room/Tool
Access Control and Machine Guarding.
Refer to section 9.0 of the PSU Shop Safety Program for more information. The program can be
found here: http://www.ehs.psu.edu/occhealth/safety.cfm
4
6

4
7
6.0 CHEMICAL HAZARDS
"What is it that is not poison? All things are poison, and nothing is without poison. It is the
dose only that makes a thing not a poison."
-- Paracelsus (1493 - 1541)
6. 1 Hazard Communication
The United States has adopted the United Nations Globally Harmonized System of Classification and
Labeling of Chemicals (GHS). The GHS is a comprehensive approach to defining a chemical’s hazards
and communicating those hazards and protective measures to workers.
Pictograms identify health, physical and environmental hazards associated with a chemical.
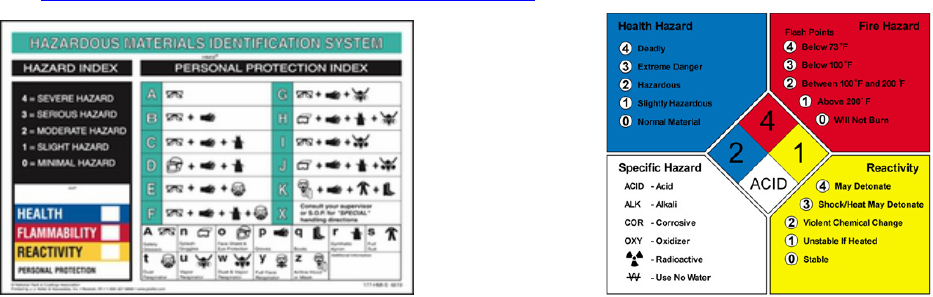
4
8
6.1.1 Safety Data Sheets (SDSs)
SDSs are the most basic source of chemical hazard information. The SDS summarizes the
chemical’s properties, the health and physical hazards, including the type of toxicity information
discussed in the sections below, and related safety information required by emergency
responders.
PIs or supervisors shall provide staff with easy access to SDSs for each of the chemicals in use or
storage in their labs. Contact EHS for help in obtaining access to SDSs.
6.1.2 Chemical Labels
Hazardous chemicals must have labels which include:
Product identifier: name or number used for a hazardous chemical on the label or SDS.
Signal word: a word used to indicate the relative severity of the hazard and alert the
reader to a potential hazard on the label. “Danger” is used for the more severe hazards,
while “warning” is used for the less severe hazards.
Pictogram: a graphic symbol intended to convey specific information about the hazards
of a chemical.
Hazard statement: describes the nature of the hazard(s) of a chemical, including, where
appropriate, the degree of hazard. Includes hazard class, which describes the nature of the
physical or health hazard (e.g., flammable solid, carcinogen)
All containers of chemicals in the laboratory or research area shall be labeled. See Figure 6.1 for an
example of a standard hazard warning labels used by manufacturers and others throughout the
country.
When labeling workplace chemical bottles such as squirt bottles or working solutions, it is
acceptable to use standard labels such HMIS or NFPA (see examples below). Copying the
manufacturer’s lab and applying it to the container is also an option. However, the employees must
be
fully aware of the hazards of the chemicals used.” OSHA brief on Hazard
Communication: Labels and Pictograms,
https://www.osha.gov/Publications/OSHA3636.pdf
4
9
The standard recognizes the use of alternative in-plant labeling systems such as the HMIS
(Hazardous Material Information System), NFPA (National Fire Protection Association), and
others which may be used in industry. These systems rely on numerical and/or alphabetic codes
to convey hazards and are generally non-specific. OSHA has permitted these types of in-plant
labeling systems to be used when an employer’s overall HCS program is proven to be effective
despite the potential absence of target organ information on container labels. Under these
circumstances, the employer should assure – through more intensified training – that its
employees are fully aware of the hazards of the chemicals used. Additionally, employers must
ensure that their training program instructs employees on how to use and understand the
alternative labeling systems so that employees are aware of the effects (including target organ
effects) of the hazardous chemicals to which they are potentially exposed. CSHOs should
determine whether workers can recognize what hazards correspond to what code
ratings/symbols. This can be achieved through employee interviews.
Employers using alternative labeling systems must ensure that their employees are aware of all
information required to be conveyed under the HCS. OSHA will make a plant-specific
determination of the effectiveness of the complete program when an inspection is conducted. Any
employer who relies on one of these types of alternative labeling systems, instead of using labels
containing complete health effects information will – in any enforcement action alleging the
inadequacy of the labeling system – bear the burden of establishing that it has achieved a level
of employee awareness which equals or exceeds that which would have been achieved if the
employer had used labels containing complete health effects information (59 F.R. 6156).
The key to evaluating the effectiveness of any alternative labeling method is to determine
whether employees can correlate the visual warning on the in-plant container with the
applicable chemical and its appropriate hazard warnings. The alternative labeling system must
also be readily accessible to all employees in their work area throughout each work shift. For
purposes of this provision, the term “other such written materials” does not include material
safety data sheets used in lieu of labels.
- See more at: http://www.msdsonline.com/blog/2013/05/nfpa-hmis-and-osha%E2%80%99s-
ghs-aligned-hazard-communication-standard/#sthash.rn6xuvng.dpuf

5
0
FIG. 6.1
6.2 Exposure to Chemicals
A thorough discussion of toxicity is beyond the scope of any single publication. Individuals who handle
chemicals should supplement the information in this manual with specific details applicable to their
laboratories. Safety Data Sheets and other reference materials are available electronically on the EHS
website, www.ehs.psu.edu and in hard copy by request from the EHS office.
The complex relationship between a material and its biological effect in humans involves considerations of
dose, duration and frequency of the exposure, route of exposure, and many other factors, including gender,
allergic factors, age, previous sensitization, and lifestyle.
6.2.1 Exposure Routes
Chemicals enter the body through the following routes:
Inhalation -- absorption through the respiratory tract by inhalation. This is probably the easiest
way for chemicals to enter the body.
Ingestion -- absorption through the digestive tract by eating or smoking with contaminated
hands or in contaminated work areas. Depending on particle or droplet size, aerosols may also
be ingested.
Skin or eye contact -- absorption through the skin or eyes. Skin contact is the most common
cause of the widespread occupational disease dermatitis. The eyes are very porous and can
easily absorb toxic vapors that cause permanent eye damage.
5
1
Injection -- percutaneous injection through the skin. This can occur through misuse of sharp
items, especially hypodermic needles. Toxic effects can be immediate or delayed, reversible or
irreversible, local or systemic.
6.2.2 Acute and Chronic Toxicity
Toxicity is the measure of a poisonous material's adverse effect on the human body or its ability to
damage or interfere with the metabolism of living tissue. Generally, toxicity is divided into two
types, acute and chronic. Many chemicals may cause both types of toxicity, depending on the
pattern of use.
Acute toxicity is an adverse effect with symptoms of high severity coming quickly to a crisis.
Acute effects are normally the result of short-term exposures and are of short duration. Examples of
acutely toxic chemicals are hydrogen cyanide and ammonia.
Chronic toxicity is an adverse effect with symptoms that develop slowly over a long period of time
as a result of frequent exposure. The dose during each exposure period may frequently be small
enough that no effects are noticed at the time of exposure. Chronic effects are the result of long-
term exposure and are of long duration. Carcinogens as well as many metals and their derivatives
exhibit chronic toxicity.
Cumulative poisons are chemicals that tend to build up in the body as a result of numerous chronic
exposures, leading to chronic toxicity. The effects are not seen until a critical body burden is
reached. Examples of cumulative poisons are lead and mercury.
With substances in combination, such as exposure to two or more hazardous materials at the same
time, the resulting effect can be greater than the combined effect of the individual substances. This
is called a synergistic or potentiating effect. One example is concurrent exposure to alcohol and
chlorinated solvents.
The published toxicity information for a given substance is general--human data may not be
available--and the actual effects can vary greatly from one person to another. Do not underestimate
the risk of toxicity. All substances of unknown toxicity should be handled as if they are toxic, with
the understanding that any mixture may be more toxic than its most toxic component.
6.2.3 Carcinogenicity
A carcinogen is a chemical that causes malignant (cancerous) tumors. A list of
substances NIOSH considers to be potential occupational carcinogens are listed in Table
6.1 along with recognized and suspected carcinogens identified by other agencies.
6.2.4 Reproductive Toxins
Chemicals can affect both adult male and female reproductive systems. Chemicals may also affect
a developing fertilized ovum, embryo, or fetus through exposure to the mother (teratogenic effects).
Reproductive hazards affect people in a number of ways, including mental disorders, loss of sexual
5
2
drive, impotence, infertility, sterility, mutagenic effects on cells, teratogenic effects on the fetus, and
transplacental carcinogenesis. Consult the SDS for information on possible reproductive hazards.
Table 6.2 provides a list of developmental and reproductive toxins.
6.2.5 Designated Area
Work involving selected carcinogens, reproductive toxins, and substances of high acute toxicity
shall be conducted in a "designated area." This area shall be so posted, and all employees working
within the area shall be informed of the hazardous substances used there. The designated area may
be a chemical fume hood, a part of a laboratory, or the entire laboratory.

5
3
TABLE 6.1 NIOSH CARCINOGEN LIST
The following is a list of substances NIOSH considers to be potential occupational carcinogens.
A number of the carcinogen classifications deal with groups of substances: aniline and homologs, chromates,
dintrotoluenes, arsenic and inorganic arsenic compounds, beryllium and beryllium compounds,
cadmium compounds, nickel compounds, and crystalline forms of silica. There are also substances of
variable or unclear chemical makeup that are considered carcinogens, coal tar pitch volatiles,
coke oven emissions, diesel exhaust and environmental tobacco smoke.
Some of the potential carcinogens listed in this index may be re-evaluated by NIOSH as new data become
available and the NIOSH recommendations on these carcinogens either as to their status as a
potential occupational carcinogen or as to the appropriate recommended exposure limit may change.
Acetaldehyde
2-Acetylaminofluorene
Acrylamide
Acrylonitrile
Aldrin
4-Aminodiphenyl
Amitrole
Aniline and homologs
o-Anisidine
p-Anisidine
Arsenic and inorganic arsenic compounds
Arsine
Asbestos
Asphalt fumes
Benzene
Benzidine
Benzidine-based dyes
Beryllium
Butadiene
tert-Butyl chromate; class, chromium hexavalent
Cadmium dust and fume
Captafol
Captan
Carbon black (exceeding 0.1% PAHs)
Carbon tetrachloride
Chlordane
Chlorinated camphene
Chlorodiphenyl (42% chlorine); class polychlorinated
biphenyls
Chlorodiphenyl (54% chlorine); class polychlorinated
biphenyls
Chloroform
Chloromethyl methyl ether
bis(Chloromethyl) ether
B-Chloroprene
Chromium, hexavalent [Cr(VI)]
Chromyl chloride; class, chromium hexavalent
Chrysene
Coal tar pitch volatiles; class, coal tar products
Coke oven emissions
DDT (dichlorodiphenyltrichloroethane)
Di-2-ethylhexyl phthalate (DEHP)
2,4-Diaminoanisoleo
o-Dianisidine-based dyes
1,2-Dibromo-3-chloropropane (DBCP)
Dichloroacetylene
p-Dichlorobenzene
3,3'-Dichlorobenzidine
Dichloroethyl ether
1,3-Dichloropropene
Dieldrin
Diesel exhaust
Diglycidyl ether (DGE); class, glycidyl ethers
4-Dimethylaminoazobenzene
Dimethyl carbomoyl chloride
1,1-Dimethylhydrazine; class, hydrazines
Dimethyl sulfate
Dinitrotoluene

5
4
Dioxane
Environmental tobacco smoke
Epichlorohydrin
Ethyl acrylate
Ethylene dibromide
Ehtylene dichloride
Ethylene oxide
Ethyleneimine
Ethylene thiourea
Formaldehyde
Gallium arsenide
Gasoline
Heptachlor
Hexachlorobutadiene
Hexachloroethane
Hexamethyl phosphoric triamide (HMPA)
Hydrazine
Kepone
Malonaldehyde
Methoxychlor
Methyl bromide; class, monohalomethanes
Methyl chloride
Methyl iodide; class, monohalomethanes
Methyl hydrazine; class, hydrazines
4,4'-Methylenebis(2-chloroaniline) (MBOCA)
Methylene chloride
4,4-Methylenedianiline (MDA)
a-Naphylamine
B-Naphylamine
Nickel, metal, soluble, insoluble, and inorganic;
class, nickel, inorganic
Nickel carbonyl
Nickel sulfide roasting
4-Nitrobiphenyl
p-Nitrochlorobenzene
2-Nitronaphthalene
2-Nitropropane
N-NitrosodimethylaminePentachloroethane; class,
chloroethanes
N-Phenyl-b-naphthylamine; class, b-naphthalene
Phenyl glycidyl ether; class, glycidyl ethers
Phenylhydrazine; class, hydrazines
Propane Sultone
B-Propiolactone
Propylene dichloride
Proplyene imine
Propylene oxideRadon
Rosin core solder, pyrolysis products (containing
formaldehyde)
Silica, crystalline cristobalite
Silica, crystalline quartz
Silica, crystalline tripoli
Silica, crystalline tridymite
silica, fused
Soapstone, total dust silicatesTremolite silicates
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD)
(dioxin)
1,1,2,2-Tetrachloroethane
Tetrachloroethylene
Titanium dioxide
o-Tolidine-based dyes
o-Tolidine
Toluene diisocyanate (TDI)
Toluene diamine (TDA)
o-Toluidine
p-Toluidine
1,1,2-Trichloroethane; class, chloroethanes
Trichloroethylene
1,2,3-TrichloropropaneUranium, insoluble
compounds Uranium, soluble compounds
Vinyl bromide; class, vinyl halides
Vinyl chloride
Vinyl cyclohexene dioxide
Vinylidene chloride (1,1-dichloroethylene); class,
vinyl halides)
Welding fumes, total particulates
Wood dust
Zinc chromate; class, chromium hexavalent

5
5
TABLE 6.2 Reproductive Toxins
This list was produced from the State of California Safe
Drinking Water and Toxic Enforcement Act (Proposition 65)
list of chemicals known to the State to cause reproductive
toxicity. Developmental toxins:
Chemical CAS Number
acetohydroxamic acid 546883
all-trans retinoic acid 302794
alprazolam 28981977
amikacin sulfate 39831555
aminoglutethimide 125848
aminopterin 54626
benomyl 17804352
benzphetamine hydrocholoride 5411223
bischloloroethyl nitrosourea (BNCU) (Carmustine) 154938
bromoxynil 1689845
1,4-butanediol dimethylsulfonate (Busulfan) 55981
carbon disulfide 75150
carboplatin 41575944
chenodiol 474259
chlorcyclizine hydrochloride 1602219
chlorambucil 305033
chlordecone (Kepone) 143500
1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU) 13010474
clomiphene citrate 50419
conjugated estrogens
cyanazine 21725462
cycloheximine 66819
cyclophosphamide (anhydrous) 50180
cyclophosphamide (hydrated) 6055192
cyhexatin 13121705
cytarabine 147944
danazol 17230885
daunorubiciin hydrocholoride 23541506
diethylstilbestrol (DES) 56531
dinocap 39300453
dinoseb 88857

5
6
diphenylhydantoin (phenytoin) 54410
doxycycline 564250
ergotamine tartrate 379793
ethylene glycol monoethyl ether 110805
ethylene glycol monomethyl ether 109864
etoposide 33419420
etretinate 54350480
fluorouracil 51218
fluoxymesterone 76437
flutamide 13311847
halazepam 23092173
hexachlorobenzene 118741
ifosfamide 3778732
iodine-131 24267569
isotretinoin 4759482
lead 7439921
lithium carbonate 554132
lithium citrate 919164
medroxyprogesterone acetate 71589
megestrol acetate 595335
melphalan 148823
menotropins 9002680
mercaptopurine 6112761
mercury and mercury compounds 7439976
methacycline hydrochloride 3963959
methimazole 60560
methotrexate 59052
methotrexate sodium 15475566
methyl mercury 593748
methyltestosterone 58184
midazolam hydrochloride 59467968
misoprostol 62015398
mitroxantrone hydrochloride 70476823
nafarelin acetate 86220420
netilmicin sulfate 56391572
nitrogen mustard (mercloroethamine) 51752

5
7
nitrogen mustard hydrochloride (mechlorethamine hydrochloride) 55867
norethisterone (norethindrone) 68224
norethisterone (norethindrone)/ethinyl estradiol 68224/57636
norethisterone (norethindrone)/mestranol 68224/72333
norgestrel 6533002
oxytetracycline 79572
paramethadione 115673
penicillamine 52675
pentobarbital sodium 57330
phenacemide 63989
pipobroman 54911
plicamycin 18378897
polychlorinated biphenyls
procarbazine hydrocholride 366701
propylthiouracil 51525
streptomycin sulfate 3810740
tamoxifen citrate 54965241
temazepam 846504
testosterone enanthate 315377
2,3,7,8-tetrachlorodibenzo-para-dioxin (TCDD) 1746016
tetracycline hydrochloride 64755
thalidomide 50351
thioguanine 154427
tobramycin sulfate 49842071
toluene 108883
triazolam 28911015
trilostane 13647353
trimethainone 127480
urofollitropin 26995915
valproate 99661
vinblastine sulfate 143679
vinvristine sulfate 2068782
warfarin 81812

5
8
2. Female reproductive toxins:
Chemical CAS Number
aminopterin 54626
anabolic steroids
carbon disulfide 75150
cyclophosphamide (anhydrous) 50180
cyclophosphamide (hydrated) 6055192
ethylene oxide 75218
lead 7439921
3. Male reproductive toxins:
Chemical CAS Number
anabolic steroids
benomyl 178043252
carbon disulfide 75150
cyclophosphamide (anhydrous) 50180
cyclophosphamide (hydrated) 6055192
1,2-dibromo-3-chloropropane 96128
m-dinitrobenzene 99650
o-dinitrobenzene 528290
p-dinitrobenzene 100254
dinoseb 88857
ethylene glycol monoethyl ether 110805
ethylene glycol monomethyl ether 109864
lead 7439921
5
9
6.2.6 Monitoring Airborne Concentrations of Contaminants
OSHA has established permissible exposure limits (PELs) for airborne concentrations of selected
materials. The PEL is defined as a time-weighted average (TWA) concentration of a particular
substance for a normal 8-hour workday and a 40-hour workweek, a concentration to which nearly
all workers (except those with particular sensitivities) may be exposed, day after day, without
adverse effect.
Corollaries to the 8-hour PEL are the short-term exposure limit (STEL) and the ceiling exposure
limit. The STEL is the time-weighted average concentration of a compound to which a worker may
be exposed over a period of 15 minutes without expecting symptoms of irritation, chronic or
irreversible tissue damage, or narcosis. The ceiling is the concentration of a substance that should
not be exceeded during any part of the working exposure. In conventional industrial hygiene
practice, the ceiling limit is also assessed over 15 minutes.
As the PELs were designed to protect workers in industrial settings, it is unlikely that these limits
will be exceeded during the performance of laboratory procedures. Laboratory workers do not
generally handle the same quantities of hazardous materials as do manufacturing and production
employees.
Nonetheless, exposure to airborne chemicals in laboratories shall not exceed PELs. If there is
reason to believe that airborne concentrations may exceed PELs, contact EHS for consultation on
the need for air monitoring. PELs are listed on Safety Data Sheets or are available from EHS.
Please note that PELs have not been developed for all the compounds to which laboratory workers
may be exposed. In all circumstances, caution shall be used in handling hazardous chemicals.
In addition to PELs, OSHA has set action levels for specific compounds, such as formaldehyde,
cadmium, and lead, for which individual standards have been promulgated. OSHA has classified
these compounds as potential carcinogens. Action levels are concentrations of a chemical in air at
which OSHA regulations to protect workers take effect.
If monitoring of airborne concentrations reveals that levels are above the OSHA action level, then
levels shall either be immediately reduced by a procedural change or equipment modification or the
department head and principal investigator shall comply with the requirements of the OSHA
standard for the chemical. OSHA regulations govern periodic monitoring and termination of
monitoring, as well as employee notification. Medical surveillance may be a requirement.
For chemicals without regulated action levels, the general rule is that half the PEL may be
considered a de facto action level. Engineering controls shall be instituted to reduce exposure to the
hazardous substance in question.
6.3 Guidelines for Handling Chemicals
The chemical handling guidelines described in this document are founded on several basic principles:
6
0
Minimize chemical exposures
Avoid underestimating risk
Provide adequate ventilation
Since most chemicals are hazardous to some degree, it is prudent to minimize exposure to chemicals as a
general rule, rather than implementing safety protocols only for specific compounds. Avoid skin contact
with chemicals as much as possible. Assume that mixtures are more toxic than their components and that
all substances of unknown toxicity are toxic. Do not work with a volatile or aerosolizing material without
adequate ventilation from chemical fume hoods or other protective devices. Remember: Prepare yourself,
then protect yourself.
6.3.1 General Guidelines
The guidelines in Figure 6.1 are applicable to nearly all uses of chemicals in laboratories. They
apply to most hazardous chemicals, such as acids, bases, and flammable liquids. They are also
applicable to chemicals that display low carcinogenic potency in animals and are not considered
carcinogens.
The general guidelines are not, by themselves, adequate for chemicals with high acute toxicity or
high chronic toxicity such as heavy metals, chemical carcinogens, or reproductive toxins.

6
1
FIG. 6.2 GENERAL GUIDELINES FOR CHEMICAL HANDLING
Wear gloves selected on the basis of the hazard. Inspect them before use. Wash reusable
gloves before removal. Turn disposable gloves inside out carefully to avoid contaminating
hands.
Always wash hands, and possibly arms and face, before leaving the laboratory, eating,
smoking, drinking, chewing gum, applying lip balm or cosmetics, or handling contact lenses.
Do not store or prepare food, eat, drink, chew gum, apply lip balm or cosmetics, or handle
contact lenses in areas where hazardous chemicals are present.
Wear eye protection at all times where chemicals are used or stored.
Never pipette or start a siphon by mouth.
Never smell or taste chemicals.
Vent into local exhaust devices any apparatus that may discharge toxic vapors, fumes, mists,
dusts, or gases. Never release toxic chemicals into cold rooms or warm rooms that have
recirculating atmospheres.
Use chemical fume hoods or other engineering controls to minimize exposure to airborne
contaminants. Use a respirator only if engineering controls are not adequate.
Keep work areas clean and uncluttered.
Label all chemical containers.
Obtain an SDS for each chemical, and consult the SDS before you use a chemical.
Confine long hair and loose clothing.
Wear sturdy shoes that cover feet completely.
Wear a laboratory coat or other protective clothing. Remove protective clothing
immediately if it becomes significantly contaminated.
Know the emergency procedures for the building, the department, and the chemicals being
used.
Keep personal belongings away from chemicals
6
2
6.3.2 Guidelines for Working with Chemicals of Acute Toxicity
Chemicals of acute toxicity are defined by OSHA as those that cause rapid effects as a result of a
short-term exposure--generally sudden and severe, as in the case of a leak from equipment. Acute
toxic effects include irritation, corrosion, sensitization, and narcosis.
To illustrate, hydrofluoric acid is a chemical of high acute toxicity because of its destructive effect
on skin and bone tissue. Arsine and other hydrides may be lethal at low concentrations because of
red blood cell hemolysis. Inhalation of high concentrations of carbon monoxide can cause
immediate poisoning and death, as the gas directly interferes with oxygen transport in the body by
preferentially binding with hemoglobin. Hydrogen cyanide inhalation inhibits enzyme systems vital
to cellular uptake of oxygen.
When working with significant quantities of such chemicals, the aim is to minimize exposure to the
material in use and to minimize the effects of exposure. Special care should be taken in the
selection of protective clothing to ensure it is appropriate for the hazard. Personal hygiene and
work practices should also be carefully evaluated to minimize exposure. The following guidelines
should be practiced in addition to the general guidelines for handling chemicals.

6
3
FIG. 6.3 GUIDELINES FOR HANDLING ACUTELY TOXIC CHEMICALS
Wear lab coat, gloves, and appropriate eyewear.
When performing procedures that may result in the release of airborne contaminants,
use a chemical fume hood with adequate draw or other protective ventilation
devices, such as snorkels or hard-piped cabinet or equipment-exhaust ventilation.
Trap or treat effluents to remove gases, fumes, vapors, and particulates before discharging
them to facility exhaust.
Restrict access to the laboratory or work area.
Establish and label a "designated area" for work with acutely toxic chemicals. Keep
materials within the designated area.
Use plastic-backed paper or trays under work areas. Replace the paper when contaminated.
Develop and know special emergency procedures. Keep emergency supplies at hand for
immediate use.
Store and dispose of chemical wastes safely.
6
4
6.3.3 Guidelines for Working with Pesticides
In addition to the general guidelines for working with chemicals, the following are specific to pesticide use.

6
5
FIG. 6.4 Guidelines for Working with Pesticides
Pesticide applications must be made at licensed facilities by certified applicators. For the
rare exceptions to this requirement, please contact EHS.
Properly store pesticides. Liquid pesticides are required to have secondary containment.
The storage of pesticides should be kept to a minimum. Pesticide storage area must be
posted with this warning sign:
Provide a spill kit, fire extinguisher, and first aid kit near all pesticide storage facilities.
Provide all label required PPE for all of the pesticides within the storage area. Keep this
PPE separate from the pesticides.
Provide decontamination supplies (See Section 5.4.5)
The label is the law!
Ensure notification of hypersensitive persons within 500 feet of the application site.
Use Integrated Pest Management (IPM).
Transport of pesticides requires that the vehicle be posted with the business license
number.
Ensure that Worker Protection Standards are followed for applications used in the
production of agricultural plants on farms, forests, nurseries, and greenhouses.
Agricultural plants include those grown or maintained for commercial or research
purposes.
o Training of workers/handlers every 5 years
o Central posting location (worker safety poster, application records, emergency
information)
o Restricted entry intervals (REIs) followed
o Post pesticide applications
6
6
6.3.4 Guidelines for Chemicals with High Chronic Toxicity, Carcinogens, and
Reproductive Toxins
In addition to the general guidelines for handling chemicals, use the following guidelines for
handling chemicals with high chronic toxicity, which include most heavy metals, chemicals
displaying moderate to high carcinogenic potency in animals, and reproductive toxins.

6
7
FIG. 6.5 GUIDELINES FOR HANDLING CHEMICALS WITH HIGH CHRONIC
TOXICITY, CARCINOGENS, AND REPRODUCTIVE TOXINS
* Wear laboratory coats and have them cleaned frequently. Laboratory coats shall be removed
before leaving the laboratory; they shall not be worn outside the laboratory in hallways,
offices, conference rooms, or eating areas. The principal investigator shall decide whether
impermeable aprons or disposable laboratory coats are required.
* Select protective glove material on the basis of the chemical being handled. Remove gloves
before leaving the work area. Turn disposable gloves inside out when removing them.
Wash reusable gloves before removing them.
* Wash hands and arms immediately after working with toxic materials or carcinogens, even
though you wore gloves.
* Cover laboratory surfaces, including hood surfaces, with plastic-backed paper or protective
trays. Inspect work surfaces following procedures, and remove the paper if contamination is
present.
* Perform transfers of toxic or carcinogenic substances in a controlled area, such as a hood.
Weigh materials on a balance only in closed containers. Procedures generating either solid
or liquid airborne contaminants or involving volatile chemicals are always to be performed
in a hood.
* Transport highly toxic or carcinogenic materials through public areas, such as hallways, in
closed containers within unbreakable outer containers. Sealed plastic bags may be used as
secondary containment in many cases.
* Eating, drinking, smoking, gum or tobacco chewing, application of lip balm or cosmetics,
handling of contact lenses, and food storage in laboratories shall be prohibited.
* Cupboards, cabinets, hoods, and refrigerators used to store or handle carcinogens shall be
labeled "Chemical Carcinogen." If they are used for chemicals with high chronic toxicity or
reproductive toxins, they shall be labeled "Toxic Chemical" or "Toxic Substance."
Continued on next page.

6
8
FIG. 6.5 GUIDELINES FOR HANDLING CHEMICALS WITH HIGH CHRONIC
(CONTD.) TOXICITY, CARCINOGENS, AND REPRODUCTIVE TOXINS
* Access to areas where carcinogens or chemicals with high chronic toxicity are used shall be
restricted, and entry doors shall be kept closed. Doors shall be labeled "Cancer-Suspect
Agent: Authorized Personnel Only" or "Toxic Chemical (or Toxic Substance): Authorized
Personnel Only."
* Establish a designated area within the laboratory for use of the materials. Label the area.
Materials shall be kept within the designated area to the extent possible.
* To avoid potential inhalation hazards, handle powdered carcinogens and toxins in a hood,
even during weighing procedures. Inside the hood, measure the powder with a spatula into a
preweighed vessel. Then seal or cover the vessel, remove it from the hood, and take it to the
balance to be weighed. If more or less material is needed, return the container to the hood
for addition or subtraction of material. Close the container again and reweigh it. Repeat
these steps until the desired amount is obtained. This procedure eliminates contamination of
the air, the work bench, and the scale.
* In the event that a worker is exposed to a known chemical, the worker shall follow general or
chemical-specific first aid procedures for chemical spills. Wash the affected area or take a
shower as soon as possible and notify EHS. See the emergency information in this section.
* Vacuum pumps shall be protected against contamination (e.g., traps and filters in lines) and
vented into direct exhaust ventilation. Pumps and other equipment and glassware shall be
decontaminated before they are removed from the designated area. The designated area shall
be decontaminated before other normal work is conducted. Vacuum pump oil shall be
collected as a contaminated waste and disposed of through EHS.
* Water vacuum lines shall be equipped with traps to prevent vapors from entering the
wastewater stream.
* Floors shall be wet-mopped or cleaned with a high-efficiency particulate air filter (HEPA)
vacuum cleaner if powdered materials are used.
* Wastes, potentially contaminated gloves and other protective equipment, glassware, and
equipment used with material of high chronic toxicity shall be kept within the designated
area to the extent possible. Decontaminate materials and equipment to the extent possible
before moving them out of the designated area.

6
9
6.4 Chemical Emergency Procedures
FIG. 6.6 PROCEDURE FOR SPILLS OF VOLATILE, TOXIC, OR FLAMMABLE
MATERIALS
* Warn all persons nearby.
* Turn off any ignition sources such as burners, motors, and other spark-producing equipment.
* Leave the room and close the door if possible.
* Call EHS and UP and go to a safe location to wait for help.
Small spills can be absorbed with paper towels or other absorbents. However, these materials
can increase the surface area and evaporation rate, increasing the potential fire hazard if the
material is flammable and airborne concentration reaches the flammability level.

7
0
FIG. 6.7 PROCEDURE FOR CHEMICAL SPILL ON A PERSON
* Be prepared. Keep appropriate spill-containment material on hand for emergencies. Consult
with EHS to determine which materials are suitable in a particular lab.
* Know where the nearest eyewash and safety shower are located.
* For small spills on the skin, flush immediately under running water for at least 15 minutes,
removing any jewelry that might contain residue. If there is no sign of a burn, wash the area
with soap under warm running water.
If pain returns after the 15-minute flooding, resume flooding the area. When providing
assistance to a victim of chemical contamination, use appropriate personal protective
equipment.
* For a chemical splash in the eyes, immediately flush the eyes under running potable water
for 15 minutes, holding the eyes open and rotating the eyeballs. This is preferably done at
an eyewash fountain with tepid water and properly controlled flow. Hold the eyelids open
and move the eye up, down, and sideways to ensure complete coverage. If no eyewash
fountain is available, put the victim on his or her back and gently pour water into the eyes for
15 minutes or until medical personnel arrive.
* For spills on clothing, immediately remove contaminated clothing, including shoes and
jewelry, while standing under running water or the safety shower. When removing shirts or
pullover sweaters, be careful not to contaminate the eyes. Cutting off such clothing will help
prevent spreading the contamination. To prepare for emergencies, shears (rounded-tip
scissors) should be available in the first aid kit to allow safe cutting of contaminated clothing.
* Consult the SDS to see if any delayed effects should be expected, and keep the SDS with the
victim. Have the victim taken to the emergency room for medical attention. Be sure to
inform emergency personnel of the decontamination procedures used prior to their arrival
(for example, washing for 15 minutes with water). Be certain that emergency room
personnel are told exactly what the victim was contaminated with so they can treat the victim
accordingly.

7
1
FIG. 6.8 PROCEDURE FOR CRYOGENIC LIQUID SPILL ON A PERSON
Contact with cryogenic liquids may cause crystals to form in tissues under the spill area, either
superficially or more deeply in the fluids and underlying soft tissues. The first aid procedure for
contact with cryogenic liquids is identical to that for frostbite. Rewarm the affected area as
quickly as possible by immersing it in warm, but not hot, water (between 102
o
and 105
o
F). Do
not rub the affected tissues. Do not apply heat lamps or hot water and do not break blisters.
Cover the affected area with a sterile covering and seek assistance as you would for burns.

7
2
FIG. 6.9 PROCEDURE FOR SMALL, LOW-TOXICITY CHEMICAL SPILLS*
* Alert persons in the area that a spill has occurred.
* Evaluate the toxicity, flammability, and other hazardous properties of the chemical as well as
the size and location of the spill (for example, chemical fume hood or elevator) to determine
whether evacuation or additional assistance is necessary. For large or toxic spills beyond
your ability to control, call 911.
* Contain any volatile material within a room by keeping doors closed. Increase exhaust
efficiency by minimizing sash height of chemical fume hood.
* Consult your SDS or procedures in this document, or call EHS for correct cleaning
procedures.
* Wear protective equipment such as goggles, apron, laboratory coat, or gloves. Base the
selection of the equipment on the hazard.
* Absorb liquid spills using paper towels, spill pillows, vermiculite, or sand. Place the spill
pillow over the spill and draw the free liquid into the pillow. Sprinkle vermiculite or sand
over the surface of the free liquid.
* Place the used pillows or absorbent materials in plastic bags for disposal along with
contaminated disposable gear, such as gloves.
* Neutralize spills of corrosives and absorb, if appropriate. Sweep up waste and place in
plastic bags for disposal.
* Complete a Chemical Request Form on the EHS website, www.ehs.psu.edu. EHS will pick
up the wastes.
* Complete an incident investigation to determine cause and preventive measures for
reoccurrence.
* Information for specific chemicals may be found in Table 6.3, "Quick Reference for Spill
Cleanups," and Fig. 6.8, "Mercury Spill Procedure." Consult the SDS and your laboratory's Unit
Specific Plan, which has specific information on spill procedures for your workplace.

7
3
TABLE 6.3 QUICK REFERENCE FOR SPILL CLEANUPS
CHEMICAL SPILLED CLEANUP
Acids, organic Apply sodium bicarbonate. Absorb with spill pillow or
vermiculite.
Acids, inorganic Apply sodium bicarbonate/calcium oxide or sodium
carbonate/calcium oxide. Absorb with spill pillow or vermiculite.
Note: Hydrofluoric acid is an exception to this general practice;
see below.
Acid chlorides Do not use water. Absorb with sand or sodium bicarbonate.
Aldehydes Absorb with spill pillow or vermiculite.
Aliphatic amines Apply sodium bisulfite. Absorb with spill pillow or vermiculite.
Aromatic amines Absorb with spill pillow or vermiculite. Avoid skin contact or
inhalation.
Aromatic halogenated amines Absorb with spill pillow or vermiculite. Avoid skin contact or
inhalation.
Azides (potential explosives) Absorb with spill pillow or vermiculite. Decontaminate with 10%
ceric ammonium nitrate solution.
Bases (caustic alkalis) Neutralize with acid or commercial chemical neutralizers and
absorb with spill pillow or vermiculite.
Carbon disulfide (flammable Absorb with spill pillow or vermiculite.
and toxic)
Chlorohydrins Absorb with spill pillow or vermiculite. Avoid skin contact or
inhalation.
Cyanides Wet or mist solids before sweeping, or use a HEPA filter vacuum
to collect the solids. Absorb liquids with spill pillow or
vermiculite.
Halides, organic or inorganic Apply sodium bicarbonate.
Halogenated hydrocarbons Absorb with spill pillow or vermiculite.
Hydrazine Absorb with spill pillow or vermiculite. Avoid organic matter.

7
4
Hydrofluoric acid Absorb with calcium carbonate (or calcium oxide) rather than
sodium bicarbonate. The use of sodium bicarbonate will lead to
the formation of sodium fluoride, which is considerably more
toxic than calcium fluoride. Be careful in the choice of spill
pillows used to absorb the acid. Certain pillows contain silicates
that are incompatible with hydrofluoric acid.
Inorganic salt solutions Apply soda ash.
Mercaptans/organic sulfides Neutralize with calcium hypochlorite solution. Absorb with spill
pillow or vermiculite.
Nitriles Sweep up solids. Absorb liquids with spill pillow or vermiculite.
Nitro compounds, organic nitros Absorb with spill pillow or vermiculite. Avoid skin contact or
inhalation.
Oxidizing agents Apply sodium bisulfite.
Peroxides (react violently with Absorb with spill pillow or vermiculite.
water)
Phosphates, organic and related Absorb with spill pillow or vermiculite.
Reducing substance Apply soda ash or sodium bicarbonate.
Reference: Reagent Chemicals, MCB Manufacturing Chemists, Inc., 1981, pp. 359-402.

7
5
FIG. 6.10: MERCURY SPILL PROCEDURE
Mercury is a high-density, low-viscosity liquid at room temperature. During a spill, it can form tiny
droplets that adhere to surfaces and enter cracks and crevices. EHS has a mercury-vacuum and mercury-
vapor analyzer to clean up mercury spills. In the event of a spill, contact EHS immediately.
To minimize the spill hazard, place a splash plate beneath all mercury-containing equipment.
76
6.5 Medical Surveillance
6.5.1 When is Medical Surveillance Required?
Signs and Symptoms. Whenever an employee or student develops signs or symptoms associated with a
hazardous chemical exposure, that person shall be provided an opportunity to receive an appropriate medical
examination.
Exposure Monitoring. If exposure monitoring reveals that the airborne concentration of a chemical is
above the action level or the permissible exposure limit (if no action level is set) for a chemical regulated by
OSHA, medical surveillance shall be implemented for affected persons as prescribed in the OSHA standard
for the material.
Spills, Leaks, and Other Releases. If a spill, leak, explosion, or other occurrence results in the likelihood
of a hazardous chemical exposure, affected employees shall be provided an opportunity for a medical
consultation. The consultation will determine whether there is a need for a medical examination.
6.5.2 Medical Consultation and Evaluation
Medical consultation and evaluation shall be performed under the direct supervision of a licensed physician
without cost to the employee or student, without loss of pay, and at a reasonable time and place. For
employees, medical examinations or surveillance shall be provided through the Workers' Compensation
Program. For students, the medical program shall be administered through the University's Health Service
facilities.
The department head shall ensure that the following information is provided to the physician: the identity of
the chemical involved in the exposure, a description of conditions relating to the exposure, any quantitative
data available regarding the exposure, and a description of signs and symptoms experienced by the affected
person.
The employee shall ensure that the following information is obtained from the physician in writing:
* Recommendation for medical follow-up
* Results of the medical examination and associated tests
* Any medical condition revealed in the course of the examination that may place the affected
person at increased risk as a result of the exposure
* A statement that the physician has informed the affected person of the results of the
consultation or examination and any medical condition that may require further treatment
The physician shall not reveal specific findings or diagnoses unrelated to the chemical exposure. All
medical records shall be kept as part of an employee's or student's permanent file.

77
6.5.3 Medical Surveillance for Chemicals of High Chronic Toxicity
Routine medical surveillance may be warranted for individuals working with chemicals of high chronic
toxicity, including carcinogens.
Although no restriction of hiring can be made, candidates for work with carcinogens shall be informed of
the possibility of increased risk associated with these conditions:
* Strong family history of cancer, comprising at least two first-generation relatives from maternal and
paternal ancestry or a specific pattern of cancer incidence that can be recognized as a genetic trait
* A precancerous condition or past history of cancer
* A history of treatment with cytotoxic drugs
* A history of impaired immunity or current use of therapeutic doses of steroids or other
immunosuppressive drugs
* Concurrent pregnancy or likelihood of pregnancy during employment
Job tasks for certain workers using chemicals of high chronic toxicity should be evaluated to determine
whether these workers should be temporarily excluded from work or reassigned to less hazardous activities.
This is particularly appropriate for pregnant women or persons receiving immunosuppressive drugs or
therapy.
6.6 Chemical Storage
6.6.1 Chemical Dating
Chemicals shall be dated on receipt in the laboratory and on opening. This information provides a history of
the chemicals in each container and guides future researchers as to potential quality of the chemicals stored
in the laboratory. Providing container-opening dates is especially important for peroxide-forming chemicals
such as ethers, dioxane, and tetrahydrofuran that could pose an explosion hazard. Solutions shall be labeled
and dated when prepared. Chemicals shall be disposed of through EHS if they are past their expiration date.
6.6.2 Chemical Compatibility
Fisher Scientific provides excellent and easy-to-implement guidance on chemical storage,
http://www.fishersci.com/ecomm/servlet/cmstatic?storeId=10652&href=Scientific/researchAnalytical/Produ
ctsServices/Chemicals/Stockroom/storage_guidelines.jsp&store=Scientific
Chemicals shall be stored only with other compatible chemicals. Do not store them alphabetically, except
within a grouping of compatible chemicals. Chemical groupings are listed below, and their storage
arrangement is shown in a schematic diagram of a laboratory in Figure 6.10.

78
* Highly toxic (poisons) and habit-forming organic chemicals
* Flammable organic chemicals and organic acids
* Organic bases and other organic compounds
* Inorganic (mineral) acids and inorganic oxidizers (some additional separation may be required because
of the reactivity of these materials)
* Inorganic bases, reducers, and salts
Take into account specific chemical incompatibilities in all storage of chemicals. For example, nitric and
chromic acids are incompatible and shall not be stored together. Nitric acid and organic compounds
together present a dangerous fire risk. Carcinogenic chemicals are to be stored with other
s of a similar
grouping based on their properties.
TABLE 6.4 INCOMPATIBLE MATERIALS: GENERAL CATEGORIES
In general, chemicals or wastes in Column A are incompatible with those in Column B.
Column A
Column B
Acids
Oxidizing agents:
Chlorates
Chromates
Chromium trioxide
Dichromates
Halogens
Halogenating agents
Hydrogen peroxide
Nitrates
Nitric acid
Perchlorates
Permanganates
Peroxides
Persulfates
Bases
Metals
Reducing agents:
Ammonia, anhydrous and aqueous
Carbon
Metal hydrides
Metals
Nitrites
Organic compounds*****
Phosphorus
Silicon
Sulfur
The examples of oxidizing and reducing agents are illustrative of common laboratory chemicals and
are not intended to be exhaustive.
***** The mixing of organic compounds with oxidizers or strong acids is to be avoided.
Extremely hazardous reactions may occur.

79
From Prudent Practices for Disposal of Chemicals from Laboratories, Committee on Hazardous
Substances in the Laboratory, et al., National Academy Press, Washington, D.C., 1983.

80
TABLE 6.5 INCOMPATIBLE MATERIALS CHART
Chemical Is Incompatible With Chemical Is Incompatible With
Acetic Acid Chromic acid, nitric acid, hydroxyl compounds,
ethylene glycol, perchloric acid, peroxides,
permanganates
Fluorine Isolate from everything
Acetic anhydride Hydroxyl-containing compounds such as ethylene
glycol, perchloric acid
Hydrides Water
Acetone Concentrated nitric and sulfuric acid mixtures,
hydrogen peroxide
Hydrocarbons
(benzene, butane,
propane, gasoline,
turpentine, etc.)
Fluorine, chlorine, bromine, chromic acid, peroxides
Acetylene Chlorine, bromine, copper, fluorine, silver, mercury Hydrocyanic acid Nitric acid, alkalis
Alkali and alkaline
earth metals, such as
sodium, potassium,
lithium, magnesium,
calcium, powdered
aluminum
Carbon dioxide, carbon tetrachloride, other chlorinated
hydrocarbons (also prohibit the use of water, foam, and
dry chemical extinguishers on fires
Hydrofluoric acid
(anhydrous)
Hydrogen fluoride
Ammonia (aqueous or anhydrous)
Ammonia (anhydrous) Mercury (in manometers, for example), chlorine,
calcium hypochlorite, iodine, bromine, hydrogen
fluoride
Hydrogen
peroxide
Copper, chromium, iron, most metals or their salts, any
flammable liquid, combustible materials, aniline,
nitromethane
Ammonium nitrate Acids, metal powders, flammable liquids, chlorates,
nitrites, sulfur, finely divided organics, combustibles
Hydrogen sulfide Fuming nitric acid, oxidizing gases
Aniline Nitric acid, hydrogen peroxide Hypochlorites Acids, activated carbon
Arsenates and arsenites Any reducing agents Iodine Acetylene, ammonia (aqueous or anhydrous)
Azides Acids, heavy metals and their salts, oxidizing agents Mercury Acetylene, fulminic acid (produced in nitric acid-ethanol
mixtures), ammonia
Bromine Ammonia, acetylene, butadiene, butane, other
petroleum gases, sodium carbide, turpentine, benzene,
finely divided metals
Nitrates Acids, reducing agents
Calcium oxide Water Nitric acid
(concentrated)
Acetic acid, acetone, alcohol, aniline, chromic acid,
hydrocyanic acid, hydrogen sulfide, flammable liquids,
flammable gases, nitratable substances
Carbon (activated) Calcium hypochlorite, other oxidants Nitrites Acids, oxidizing agents
Carbon tetrachloride Sodium Nitroparaffins Inorganic bases, amines
Chlorates Ammonium salts, acids, metla powders, sulfur, finely
divided organics, combustibles
Oxalic acid Silver, mercury, and their salts
Chlorine Ammonia, acetylene, butadiene, butane, methane,
propane (or other petroleum gases), hydrogen, sodium
carbide, benzene, finely divided metals, turpentine
Oxygen Oils, grease, hydrogen, flammable materials (liquids,
solids, or gases)
Chlorine dioxide Ammonia, methane, phosphine, hydrogen sulfide Perchloric acid Acetic anhydride, bismuth and its alloys, alcohol, paper,
wood, grease, oils (all organics)
Chromic acid and
chromium trioxide
Acetic acid, naphthalene, camphor, glycerol,
turpentine, alcohol, other flammable liquids
Peroxides, organic Acids (organic or mineral); avoid friction, store cold
Copper Acetylene, hydrogen peroxide Phosphorus
(white)
Air, oxygen, alkalis, reducing agents
Cumene hydroperoxide Acids (organic or inorganic) Phosphorus
pentoxide
Alcohol, strong bases, water
Cyanides Acids Potassium Carbon tetrachloride, carbon dioxide, water
Flammable liquids Ammonium nitrate, chromic acid, hydrogen peroxide,
nitric acid, sodium peroxide, halogens
Potassium chlorate
(see also
chlorates)
Acids

81
TABLE 6.5 INCOMPATIBLE MATERIALS CHART
(CONTD.)
Chemical Is Incompatible With Chemical Is Incompatible With
Potassium perchlorate
(see also perchloric acid)
Acids Sodium nitrite Ammonium nitrate and other ammonium salts
Potassium permanganate Glycerol, ethylene glycol, benzaldehyde, sulfuric
acid
Sodium peroxide Any oxidizable substance, such as ethanol, methanol,
glacial acetic acid, acetic anhydride, benzaledehyde,
carbon disulfide, glycerol, ethylene glycol, ethyl
acetate. methyl acetate, furfural
Selenides Reducing agents Sulfides Acids
Silver and silver salts Acetylene, oxalic acid, tartaric acid, ammonium
compounds, fulminic acid (produced in nitric acid-
ethanol mixtures)
Sulfuric acid Chlorates, perchlorates, permanganates
Sodium (see also alkali
metals)
Carbon tetrachloride, carbon dioxide, water Tellurides Reducing agents
From Prudent Practices for Handling Hazardous Chemicals in Laboratories, National Research
Council, 1981.
82
6.6.3 Storage Facilities
Highly toxic chemicals (such as cyanide), shock-sensitive chemicals, and habit-forming
chemicals shall be stored in locked cabinets to prevent theft.
Peroxide-forming chemicals and those that may become shock-sensitive with long-term storage
shall be stored separately and shall be labeled and dated. Peroxide-forming chemicals shall be
stored in a cool, dark, dry place.
Flammable liquids shall be stored in flammable-liquid cabinets if the laboratory contains a total
of 10 gallons or more, including flammable liquid wastes.
Volatile or highly odorous chemicals shall be stored in a well-ventilated area; a ventilated
cabinet is preferable. Chemical fume hoods are a poor choice for storage, as containers block
proper air flow in the hood and take up work space.
Storage areas for carcinogens shall be labeled "Chemical Carcinogen." This requirement for
cancer-warning labels applies even to chemicals that exhibit more than one hazard (e.g.,
carcinogenic and flammable).

83
FIG. 6.11 EXAMPLES OF CHEMICALS STORED BY HAZARD
I. Poisons and Habit-Formers IV. Inorganic Bases, Reducers, and Salts
POISONS INORGANIC BASES
Arsenic trioxide (carcinogen) Ammonium hydroxide
Sodium azide (solid may be shock- Potassium hydroxide
sensitive) Sodium hydroxide
Sodium cacodylate (solid)
Strychnine INORGANIC REDUCERS
Sodium sulfite
HABIT-FORMERS
Sodium pentobarbital INORGANIC SALTS
Calcium chloride
II. Flammable Organics and Organic Acids Lithium carbonate
Sodium silicate
FLAMMABLE ORGANICS
Acetone V. Organic Bases and Compounds
Benzene (carcinogen)
Diethyl ether (peroxide-former) ORGANIC BASES
Ethanol Diaminopentane
Pyridine Diethylamine
Tetrahydrofuran (peroxide-former) Hexamethyleneimine
Xylene(s)
ORGANIC COMPOUNDS
ORGANIC ACIDS Dextrose
Acetic acid Ethylenediaminetetraacetic acid
Formic acid (EDTA)
Formaldehyde (carcinogen)
III. Inorganic Acids and Oxidizers Formamide
Isoleucine
INORGANIC ACIDS Naphthol
Chromic acid (separate from nitric)
Hydrochloric acid VI. Carcinogens
Hydrofluoric acid (for which specific labeling is required)
Nitric acid (separate from chromic)
Perchloric acid Aflatoxins
Sulfuric acid Benzene
Benzidine
INORGANIC OXIDIZERS Carbon tetrachloride
Ammonium persulfate (separate from 3,3'-Diaminobenzidine
sodium nitrite) Ethidium bromide (mutagen)
Chromium trioxide Hydrazine
Hydrogen peroxide Nitrosodiethylamine
Silver nitrate
Sodium nitrate

84
6.6.4 Inspection of Stored Chemicals
Storage Area. Chemical storage areas shall be inspected annually, and any unwanted or expired
chemicals shall be removed. During this inspection, the list of chemicals present in the laboratory
shall be updated or verified and the date and name of the inspector recorded.
Inspections. Although the deterioration in storage of a specific compound cannot be predicted in
detail, generalization can often be made about the reaction characteristics of groups of
compounds. Some general conclusions about the stability of classes of chemicals can be reached,
and corresponding storage time spans can be identified. Visual inspection of stored chemicals is
important in the disposal decision.
Chemicals showing any of the indications listed below shall be turned over to EHS for safe
disposal:
* Slightly cloudy liquids
* Darkening or change in color
* Spotting on solids
* Caking of anhydrous materials
* Existence of solids in liquids or liquids in solids
* Pressure buildup in containers
* Evidence of reaction with water
* Corrosion or damage to the container
6.6.5 Refrigerator Storage (PSU Safety Policy SY11 Refrigerators – Explosion Proof)
Flammable liquids shall not be stored in ordinary domestic refrigerators. Refrigerator
temperatures are almost universally higher than the flash points of the flammable liquids most
often stored in the units, and ignition sources are readily available inside the storage
compartment. Furthermore, the compressor and its circuits are typically located at the bottom of
the units, where vapors from flammable liquid spills or leaks may easily accumulate.
If refrigerators are used in a laboratory for purposes other than flammables storage, they shall be
labeled "No Flammables Allowed." Flammable liquids shall not be stored in cold rooms that do
not have explosion-proof wiring and fixtures. Such storage facilities pose explosion hazards
because the various control switches and defroster heaters can spark and ignite flammable vapors.
Chemicals stored in refrigerators or cold rooms shall be sealed and labeled with the name of the
material and the person who stored the material. Old chemicals shall be disposed of after a
specified storage period.
85
Food shall not be stored in a refrigerator used for chemical storage. The refrigerator shall be
labeled "No Food Allowed" or equivalent. Refrigerators used for food shall be marked "Food
Only" or equivalent and shall not be near the work area.
Section 6.6.6 Tanks and Drums
All planned chemical and oil storage tanks and containers that hold 55 gallons or more must be identified
to EHS in order to assess the potential for spills and releases, and to incorporate these containers and
tanks into the various safety and training requirements of Preparedness, Prevention and Contingency
(PPC) Plans, Spill Prevention, Control, and Countermeasure (SPCC) Plans, and the Storage Tank
Management Program.
Chemicals and oils that are stored improperly may be released into the environment. Good practices,
such as training, storage, and inspections can be used to help reduce the risk of spills and releases, and to
mitigate the environmental impacts of spills and releases. In addition, there are regulatory requirements
that must be satisfied.
Facilities that have storage tanks or drums (55-gallon) will need to meet storage requirements. Secondary
containment must be provided if a spill or release from the tank or drum could enter the environment
(surface water, soil, drainage ways) or flow down a drain. A double-walled tank meets the secondary
containment requirement. Facilities that store materials in drum and tank quantities are also required to
have a spill kit, first aid kit, and a fire extinguisher.
Annual training is required at locations that are part of the University’s SPCC or PPC Plans. In addition,
these plans require inspections of tanks and drums on a monthly basis to ensure that they are in good
condition and are not causing a release.
6.7 Safety for Specific Chemical Operations
6.7.1 Unattended/Overnight Operations
The "Laboratory Information" posting on the outside of the laboratory shall have current
emergency contact information.
Reactions that are left unattended for long periods of time or overnight are prime sources of fires,
floods, and explosions. Do not run equipment such as power stirrers, hot plates, heating mantles,
and water condensers overnight without fail-safe provisions such as flow monitors that will shut
down equipment in case of water supply failure, temperature monitors interlocked into the
system, or fail-safe hose connectors.
Remember that at night, emergency personnel are entirely dependent on accurate instructions and
information available at the laboratory.

86
6.7.2 Procedures for Working Alone
No one should work alone in a laboratory. More specifically, this means that someone else
should be in close proximity (down the hall) and check in periodically on the individual. When
it is not possible to have someone close by, the individual should make arrangements to call
someone every half hour to provide updates on their status.
Everyone needs to have a supervisor, or someone acting in that capacity, at all times in order to
ensure that the supervisory responsibilities listed on p. 14 and in SY1
(http://guru.psu.edu/policies/SY01.html) are followed. If a faculty member or supervisor is out of
town, someone else must be clearly assigned to take on those responsibilities and be familiar
with the activities the individual is doing, including the potential hazards of the work.
6.7.3 Extractions and Distillations
Extractions. Extractions can present a hazard because of the potential buildup of pressure from a
volatile solvent and an immiscible aqueous phase. Glass separatory funnels used in laboratory
operations are particularly susceptible to problems because their stoppers or stopcocks can be
forced out, resulting in a spill of the contained liquid. It is even possible for pressure to burst the
vessel.
To use a separatory funnel correctly, do not attempt to extract a solution until it is cooler than the
boiling point of the extractant. When a volatile solvent is used, the unstoppered separatory funnel
should first be swirled to allow some solvent to vaporize and expel some air. Close the funnel
and invert it with the stopper held in place and immediately open the stopcock to release more air
plus vapor. Do this with the hand extended around the barrel to keep the stopcock plug securely
seated.
Point the barrel of the separatory funnel away from yourself and others and vent it to the hood.
Do not vent the funnel near a flame or other ignition source. Close the stopcock, shake with a
swirl, and immediately open the stopcock with the funnel in the inverted position to vent the
vapors again. If it is necessary to use a separatory funnel larger than one liter for an extraction
with a volatile solvent, the force on the stopper may be too great, causing the stopper to be
expelled. Consider performing the extraction in several smaller batches.
Distillations. Where possible, distillation should be replaced by column purification. During
distillation, potential dangers arise from pressure buildup, commonly used flammable materials,
and the use of heat to vaporize the chemicals involved. Careful design and construction of the
distillation system is required to accomplish effective separation and avoid leaks that can lead to
fires or contamination of the work area.
It is necessary to ensure smooth boiling during the separation process and avoid bumping, which
can blow apart the distillation apparatus. Stirring the distillation mixture is the best method to
87
avoid bumping. Boiling stones are only effective for distillations at atmospheric pressure. Use
fresh boiling stones when a liquid is boiled without stirring. Do not add boiling stones or any
other solid material to a liquid that is near its boiling point, because this may cause it to boil over
spontaneously.
An electric mantle heater, a ceramic cavity heater, steam coils, or a nonflammable liquid bath are
best to provide even heating. Silicone oil or another suitable high-boiling-temperature oil can be
used on a hot plate. Hot water or steam may also be used in some cases. An extra thermometer
inserted at the center bottom of the distilling flask will warn of dangerously high temperatures
that could indicate exothermic decomposition. Do not distill or evaporate organic compounds to
dryness unless they are known to be free of peroxides.
Because superheating and bumping occur frequently during distillation using reduced pressure, it
is important that the distillation assembly is secure and the heat distributed more evenly than is
possible with a flame. Evacuate the assembly gradually to minimize the possibility of bumping.
Stirring, or using an air or nitrogen bleed tube, provides good vaporization without overheating
and decomposition.
Put a standing shield in place for protection in the event of an implosion. After finishing a
reduced-pressure distillation, cool the system, and then slowly bleed in air so as not to induce an
explosion in a hot system. Pure nitrogen is preferred to air and can be used even before cooling
the system.
In a steam distillation, minimize the accumulation of condensate in the distillation flask. The heat
of steam condensation is very high, and overfilling the flask is less likely if condensation from the
entering steam line is trapped and the flask heated or insulated to prevent excessive condensation.
6.7.4 Temperature Control
Since the rates of most reactions accelerate as the temperature increases, highly exothermic
reactions can become violent without adequate cooling. Viscous liquids transfer heat poorly and
require special precautions. Apparatus shall be assembled so that either heating or cooling can be
applied or withdrawn readily.
6.7.4.1 Oil and Sand Baths
Improper use of a hot oil or a sand bath may create serious hazards such as an overturned bath,
spatter from water falling into the bath, smoke caused by decomposition of the oil or organic
materials in the oil, and fire from overheating the oil. Baths shall not be left unattended without
a high-temperature shutoff. The oil shall be properly labeled, including information on its safe
working temperature.
6.7.4.2 Cooling Baths

88
Ice with salt may be used when ice water is not cool enough for use as a bath. Dry ice may be
used with an organic liquid. A cooling liquid ideal for use with dry ice should have nontoxic
vapors, low viscosity, no flammability, and low volatility. Although no substance is likely to
meet all these criteria, some of the better liquids are:
* Ethylene glycol or propylene glycol in a 3:2 ratio with water and thinned with
isopropyl alcohol
* Isopropyl alcohol (less flammable than other common solvents such as
acetone or butanone)
* Some glycol ethers
Either add the dry ice to the liquid or the liquid to the dry ice in small increments. Wait for the
foaming to stop before proceeding with the addition. The rate of addition can be increased
gradually as the liquid cools. Do not handle dry ice with bare hands; if skin is even slightly
moist, severe burns can result. Use dry leather gloves or suitable cryo-gloves. Wear goggles
when chipping ice.
Cryogenic coolants shall be handled in properly vented containers. Very-low-temperature
coolants may condense oxygen and cause an explosion with combustible materials. Use gloves
and a face shield; immerse the cooling object slowly to avoid too-vigorous boiling and
overflowing the coolant. Dewar flasks should be made of borosilicate glass and wrapped with
cloth-backed friction or duct tape or put in a metal enclosure to contain flying pieces in the
event of implosion.
Dewar flasks should be equipped with safety necks. The flasks should be inspected periodically
(at least once a day) to ensure that no air or ice plugs have collected in the neck opening.
Avoid pouring cold liquid onto the edge of a glass Dewar flask when filling because the flask
may break and implode. For the same reason, do not pour liquid nitrogen out of a glass Dewar
flask. Instead, use mild air pressure or a siphon. Metal or plastic Dewar-type flasks are
preferable and eliminate this problem. Never use a household Thermos bottle in place of a
Dewar flask.
6.7.5 Reduced Pressure Operations
Protect vacuum desiccators by covering them with cloth-backed friction or duct tape or
shielding them for protection in case of implosion. Vacuum lines shall be trapped and shielding
used whenever apparatus is under reduced pressure. Only chemicals being dehydrated should
be stored in a desiccator. Before opening a desiccator that is under reduced pressure, make sure
that atmospheric pressure has been restored.
Water aspirators for reduced pressure are used mainly for filtration purposes, and only
equipment that is approved for this purpose should be used. Never apply reduced pressure to a
89
flat-bottomed flask unless it is a heavy-walled filter flask designed for that purpose. Place a trap
and a check valve between the aspirator and the apparatus so that water cannot be sucked back
into the system if the water pressure falls unexpectedly during filtering. This also applies to
rotary evaporation equipment that use water aspirators for reduced pressure.
If vacuum pumps are used, place a cold trap between the apparatus and the vacuum pump so
that volatiles from a reaction or distillation do not get into the pump oil or out into the
atmosphere. Exhausts from pumps shall be vented to a hood or ventilation system. Pumps
with belt drives must be equipped with belt guards to prevent hands or loose clothing from
being caught in the belt pulley.
6.7.6 Cold Traps
Cold traps used in reduced-pressure systems should be placed in vermiculite-filled metal cans.
If this option is not possible, the cold traps should be wrapped with cloth-backed friction or duct
tape. In the event of an implosion, the tape will reduce the amount of flying glass.
Users of cold traps should be aware of the boiling points of the components and the possible
products of materials in the reduced-pressure system. For instance, argon, a common inert gas,
may condense into traps cooled with liquid nitrogen. When the cooling bath is removed, the
argon rapidly vaporizes, and the rate of pressure buildup may be too great for the gas to be
vented or pumped down. A serious explosion could occur.
6.7.7 Transporting Chemicals
The precautions that should be followed to protect laboratory and non-laboratory personnel and
facilities when you transport chemicals in University buildings are listed below.
Use secondary containers. The container-within-a-container concept will protect the primary
containers from shock during any sudden change of movement. Secondary containment is
especially important when chemicals are moved in public areas, such as hallways or elevators,
where the effects of a spill would be more severe.
Always use a sturdy cart, and make sure the cart has a low center of gravity. Carts with large
wheels are best for negotiating irregularities in floors and at elevator doors.
Do not transport incompatible chemicals together on the same cart unless separated by
secondary containment.
All chemical containers being transported shall have labels identifying the contents.
90
Transport large containers of corrosives in a chemically resistant bucket or other container
designed for this purpose.
Anticipate sudden backing up or changes in direction from others. If you stumble or fall while
carrying glassware or chemicals, try to project them away from yourself and others.
The transportation of pesticides between facilities or from agriculture suppliers must be done by
a licensed pesticide applicator. In addition, the facility’s business license number assigned by
the PA Dept. of Agriculture must be displayed on the vehicle.
6.8 Hazards of Chemical Groups
6.8.1 Corrosives: Acids and Bases
Corrosive acids and bases attack the skin and can cause permanent damage to the eyes. See
Table 6.4 for inorganic acid neutralization procedures for spills.
All the hydrogen halide acids are serious respiratory irritants. Hydrogen fluoride poses a
special danger; both its gas and solutions are toxic, and it is rapidly absorbed through the skin,
penetrating deeply into the body tissues. Contact with dilute solutions of hydrogen fluoride may
cause no pain for several hours but result in serious burns. In all cases, immediate and thorough
flushing with water and attention by a physician are necessary.
Oxyacids such as sulfuric and nitric acid have widely differing properties. Sulfuric acid is a
very strong dehydrating agent. When preparing solutions, always add acid to water and
remember that the heat of solution may produce a large increase in temperature. Nitric acid is a
strong oxidizing agent that acts rapidly and turns exposed skin yellow to brown as a denaturing
reaction occurs. Paper that has been used to wipe up nitric acid spills can ignite spontaneously
when dry and should not be thrown into a wastebasket until first rinsed with water and
neutralized.
Chromic acid is generally prepared as a cleaning solution; EHS recommends the use of
replacement cleaners without chromium, which is carcinogenic. All chromic acid waste is
collected and disposed of through EHS. For information regarding chromic acid substitutes,
contact EHS.
Perchloric acid is a powerful oxidizing agent that may react explosively with organic
compounds and other reducing agents. It shall be used only in a perchloric-acid, water-wash-
down fume hood of noncombustible construction. Perchloric acid should be handled with
extreme care and kept from organic matter to prevent a serious explosion. Beakers of fuming
perchloric acid shall be handled with tongs rather than rubber gloves. Perchloric acid hoods
shall be washed down after every perchloric acid digestion.
91
Perchloric acid containers shall be stored in glass outer containers and shall not be stored on
wood shelving, as drips or leaks may render the wood shock-sensitive. Keep perchloric acid
bottles on glass or ceramic trays that are large enough to hold all the acid if the bottle breaks.
Storage of perchloric acid containers should not exceed one year. Digest organic matter with
nitric acid before addition of perchloric acid. Never heat perchloric acid with sulfuric acid
because dehydration may produce anhydrous perchloric acid, which is explosive.
Perchlorate esters have the same shattering effect as nitroglycerine. Transition metal
perchlorates are capable of exploding. Perchlorates shall not be used without prior consultation
with EHS.
The most common bases found in laboratories include the alkali metal hydroxides and aqueous
solutions of ammonia. Sodium and potassium hydroxides are extremely destructive to both skin
and eye tissues. When concentrated solutions are prepared, the heat of solution can raise the
temperature to dangerous levels. Because ammonia solution vapors are such strong irritants,
they should be used only in a chemical fume hood.

92
TABLE 6.6 PROCEDURE FOR INORGANIC ACID NEUTRALIZATION
(DOES NOT APPLY TO CHROMIC ACID)
APPLICABLE ACIDS: Hydrochloric, sulfuric,
EQUIPMENT: Chemical fume hood, sash pulled down as far as possible
Goggles
Gloves
Lab coat, either acid resistant or with impermeable apron
pH paper, wide range
CAUTION: Wear protective clothing and work in a hood
Beware of heat and fumes generated by neutralizing acid
Add acid to water
Keep containers cool while neutralizing, using ice in the water or
in baths
Dilute concentrated acids before neutralization
1. Prepare a large amount of an ice-water-and-base solution of one of the following:
Sodium carbonate (soda ash)
Calcium hydroxide (slaked lime)
Sodium hydroxide, 5 to 10% one-molar solution is about 4% (4 grams per 100 ml)
2. Slowly stir acid (which has been diluted to about 5%) into the base solution until the pH reaches
about 5 to 10.
3. Slowly pour the neutralized solution down the drain with large amounts of water.
NOTE: The pH of solutions poured down the drain shall be between 5 and 10 to avoid violating local,
state, or federal regulations.
Reference: Prudent Practices for Disposal of Chemicals from Laboratories, Committee on
Hazardous Substances in the Laboratory, et al., National Academy Press, 1983.

93
6.8.2 Flammable and Combustible Liquids (PSU Safety Policy SY08 Storage,
Dispensing, and Use of Flammable Liquids on PSU Property)
Definitions. According to most fire codes and regulations, including those for laboratories, a
flammable liquid is a liquid with a flash point below 100
O
F and a vapor pressure not exceeding
40 psi (absolute) at 100
O
F; it is called a Class I liquid. A liquid with a flash point of 100
O
F or
greater is classified as a combustible liquid and may be referred to as a Class II or Class III liquid
(see Table 6.5).
The U.S. Department of Transportation (DOT) and the U.S. Environmental Protection Agency
(EPA) use a different definition. These agencies define flammable liquids as those with a flash
point of 140
O
F or lower and combustible liquids as those with a flash point greater than 140
o
F
but less than 200
o
F. DOT and EPA definitions apply primarily to chemicals in transit.
Flash point is the minimum temperature at which the liquid gives off vapors in sufficient
concentration to form an ignitable mixture with air. The classes of liquids are further divided into
subclasses, depending on the flash points and boiling points of the liquids. The classifications are
important because regulations governing storage and use of a liquid are largely based on the
liquid's flash point.
Flammable liquids shall be handled only in areas with no ignition sources and shall not be heated
with open flames. If flammable liquids in metal containers or equipment are transferred, the
equipment and containers shall be bonded to avoid static-generated sparks.
Storage. Flammable liquids shall not be stored in ordinary refrigerators or cold rooms. If it is
necessary to refrigerate flammable materials, explosion-proof or flammable-storage refrigerators
shall be used. Combustible liquids are less of a fire hazard, although a rise in temperature
increases their evaporation rate and the potential for ignition. If the quantity of flammable liquids
in storage exceeds 10 gallons (including liquid waste), flammable-liquid storage cabinets must be
used.
Allowable Quantities. The maximum allowable size of containers and portable tanks for
flammable and combustible liquids is shown in Table 6.8. Although the table indicates that the
maximum allowable size of glass containers for Class IA and Class IB are one pint and one quart
respectively, the liquids may be stored in glass containers of not more than one-gallon capacity if
the required liquid purity (such as ACS analytical reagent grade or higher) would be affected by
storage in metal containers or if the liquid would cause excessive corrosion of the metal
container.
Bonding and Grounding. When a flammable liquid is poured into or withdrawn from a metal
drum, the drum and the secondary container shall be electrically bonded to each other and to the
ground to avoid the possible buildup of a static charge. Only small quantities should be
transferred to a glass container. If the liquid is transferred from a metal container to glass, the

94
metal container should be grounded. Drums of flammable liquids are not permitted in
laboratories unless the quantity is necessary for daily use and is approved by EHS.
TABLE 6.7 FLAMMABLE LIQUID CLASSIFICATION
CLASS FLASH POINT (
o
F) BOILING POINT (
o
F)
IA Below 73 Below 100
IB Below 73 At or above 100
IC At or above 73, below 100 NA
II At or above 100, below 140 NA
IIIA At or above 140, below 200 NA
IIIB At or above 200 NA
TABLE 6.8 MAXIMUM ALLOWABLE SIZE OF FLAMMABLE AND
COMBUSTIBLE LIQUID CONTAINERS IN LABORATORIES
FLAMMABLE LIQUIDS COMBUSTIBLE LIQUIDS
CONTAINER CLASS IA CLASS IB CLASS IC CLASS II CLASS III
Glass 1 pint
a
1 quart
a
1 gallon 1 gallon 5 gallons
Metal (other than
DOT drums) or
approved plastic
1 gallon 5 gallons
b
5 gallons
b
5 gallons
b
5 gallons
Safety cans 2 gallons 5 gallons
b
5 gallons
b
5 gallons
b
5 gallons
Metal drum (DOT
Spec.)
Not
allowed
5 gallons
b
5 gallons
b
60 gallons
b
60 gallons
a
Glass containers of not more than one-gallon capacity are acceptable if the required purity would be
affected by storage in metal or if excessive corrosion would result from storage in metal.
b
In instructional laboratory work areas, no container for Class I or II liquids shall exceed a capacity of
one-gallon, other than safety cans which may be of two-gallon capacity.
Reference: NFPA 45, Fire Protection for Laboratories Using Chemicals, National Fire
Protection Association, 1991.

95
6.8.3 Compressed Gases (PSU Safety Policy SY25 Compressed Gas
Cylinders)
Securing Cylinders. An added hazard of toxic, oxidizing, and other hazardous gases as
well as inert gases in cylinders is the potential for accidental pressure release; a cylinder
with the valve broken off can turn into a rocket. It is important to keep cylinders secured to
the bench or wall and to keep the caps on when they are not in use. See Table 6.9 for
maximum size and quantity limitations for compressed-gas or liquified-gas cylinders in
laboratories.
Storage. When a cylinder is not in use, close the main cylinder valve tightly. Promptly
remove the regulator from an empty cylinder, replace the protective cap, and label the
cylinder by using an "empty" tag or writing on the side of the cylinder with chalk. Never
bleed cylinders completely empty; leave a slight pressure to keep contaminants out.
Transport. When transporting a cylinder, use a wheeled cylinder cart with the capped
cylinder strapped to the cart.
Connections. Threads on cylinder-valve outlet connections have been standardized by the
Compressed Gas Association and are not the same on all cylinders. This prevents
accidental mixing of incompatible gases from an interchange of connections. Never
lubricate, modify, force, or tamper with cylinder valves. Especially do not put oil or grease
on the high-pressure side of a cylinder containing oxygen, chlorine, or another oxidizing
agent. An autoignition or explosion could result.
Environmental. Do not expose cylinders to temperatures higher than 50
o
C. Some rupture
devices on cylinders release at about 65
o
C.
Toxic Gases. For the purposes of this section, the definitions of toxic gas and highly toxic
gas in the Compressed Gas Association Standard CGA P-1.1991, "Safe Handling of
Compressed Gases in Containers," can be applied. A toxic gas is one with a median lethal
concentration (LC50) of more than 200 and less than 2,000 parts per million by volume of
gas or vapor when administered by inhalation for an hour (or less if death occurs within one
hour) to albino rats weighing between 200 and 300 grams each. A highly toxic gas is
characterized by a median LC50 of 200 ppm or less under the same conditions.
Toxic gases shall be treated by absorption, wet or dry scrubbing, combustion, or
condensation via refrigeration, before being vented to hoods or other local exhaust
arrangements. Pressure-relief devices on cylinders shall be vented to a safe place. Flow-
restricting orifices are required on cylinders of toxic gases. Toxic-gas cylinders shall be
stored in continuously mechanically ventilated enclosures, with no more than three
cylinders per enclosure. Any new laboratory construction shall require gas cabinets for
96
storage of highly toxic gases. Some toxic gases may be supplied in mixtures. Purchase of
diluted toxic gas, if feasible, will serve to reduce risk.
If these alternatives are not possible, alarm systems shall be employed to monitor the toxic
gas in use. Respirators or self-contained breathing apparatus may be available in the event
of a leak. Consult the emergency plan for the given lab area to determine the action
expected during a leak situation. EHS shall be contacted for information on selection, fit
testing, and training if respirators or SCBA have been provided. No one may use
respirators on the job without prior medical approval.
6.8.3.1 Acetylene
The following special rules apply to work with cylinders of acetylene in the laboratory:
Acetylene cylinders are partially filled with acetone and should always be kept
upright. If a cylinder has been handled in a nonupright position, do not use it until it
has sat upright for at least 30 minutes.
When connecting an acetylene cylinder, be sure to use a flash arrester at the outlet of
the cylinder and the correct kind of tubing to transfer the gas. Some tubing
materials, such as copper and lead solder, form explosive acetylides.
Never exceed the pressure limit indicated by the warning red line of an acetylene
pressure gauge.
6.8.3.2 Lecture Bottles
In addition to standard precautions, the following special rules apply to work with lecture
bottles in the laboratory:
Lecture bottles shall be stored where the temperature does not exceed 50
o
C, because
unlike larger cylinders, they do not have pressure-relief devices to prevent rupturing.
Also unlike larger cylinders, lecture bottles all have identical valve threads,
irrespective of the gas contained within.
If labels and valve tags do not agree or if there is any question as to the contents of a
lecture bottle, return the unused bottle to the supplier or contact EHS. Whenever
possible, purchase lecture bottles from suppliers who will accept the return of empty
or partially empty bottles.
When transporting lecture bottles, use a cart and block the bottles to prevent rolling
and falling.

97
6.8.4 Cryogenic Liquids and Liquified Gases
The hazards of cryogenic liquids include fire or explosion, pressure buildup, embrittlement
of structural materials, asphyxiation, and destruction of living tissue on contact. Liquid
helium and liquid nitrogen may displace air and create an atmosphere without sufficient
oxygen. Fire or explosion may occur when the liquid form of flammable gases, such as
hydrogen, is used without proper management of the gaseous phase. Liquid oxygen may
produce an enriched oxygen atmosphere, which increases the flammability of ordinary
combustible materials. Enriched oxygen levels may also cause some nonflammable
materials, such as carbon steel, to burn readily.
Contact with cryogenic liquids generally causes tissue freezing and frostbite. Even brief
contacts may be intense and painful. Prolonged contact may result in blood clots.
Appropriate protective clothing, gloves, and eye protection--preferably a face shield--shall
be worn when cryogenic liquids are handled. Do not use cloth gloves, as the cryogenic
liquids can saturate them and cause more extensive cold damage to the skin.
TABLE 6.9 MAXIMUM SIZE AND QUANTITY LIMITATIONS FOR COMPRESSED
OR LIQUIFIED GAS CYLINDERS IN LABORATORIES
Flammable Gases and
Oxygen
Liquified Flammable
Gases
Gases with High
Health Hazard Rating
Maximum cylinder size
(approximate
dimensions in inches)
10 x 50 9 x 30 4 x 15
Maximum number of
cylinders per 500
square feet or less of
floor space in
nonsprinklered areas
3 2 3**
In sprinklered areas 6* 3 3**
* In instructional laboratory work areas, the total number of cylinders shall be reduced to 3
maximum-sized cylinders. Ten approximately 2" x 12" cylinders (lecture bottles) are
allowed. In other than instructional laboratories, 25 lecture bottles are permitted.
**Cylinders of all toxic gases shall be kept in a continuously mechanically ventilated hood or
other continuously mechanically vented enclosure, with no more than 3 cylinders per enclosure.
Reference: NFPA 45, Protection for Laboratories Using Chemicals, National Fire
Protection Association, 1991.
98
6.8.5 Highly Reactive Chemicals
6.8.5.1 Organic Peroxides
Organic peroxides are among the most hazardous chemicals normally handled in
laboratories As a group, they are flammable, low-power explosives and oxidizers that
are sensitive to shock, heat, sparks, friction, impact, and light. Many of them are much
more shock-sensitive than typical explosives such as TNT.
Purchase and use of peroxides shall be kept to a minimum. Unused peroxides shall not be
returned to the container. Glass containers with screw caps or glass stoppers shall not be
used. Polyethylene bottles with screw caps are acceptable. Liquid peroxides or solutions
shall be stored so that the peroxide will not freeze or precipitate, because these forms are
extremely sensitive to heat or shock. Consistent with this precaution, they shall be kept as
cold as practical to avoid decomposition.
The sensitivity of organic peroxides to heat and shock may be reduced by diluting the
peroxides with inert solvents (such as aliphatic hydrocarbons or mineral oil). However,
not all solvents are appropriate to mix with peroxides. Toluene, in particular, is known to
induce the decomposition of diacyl peroxides. Do not use acetone or other oxidizable
materials for dilution of organic peroxides.
Ceramic, Teflon, or wood spatulas shall be used. Metal spatulas will contaminate the
peroxide, which can lead to explosive decomposition. Friction, grinding, and other forms
of impact shall be avoided.
6.8.5.2 Peroxide-Forming Chemicals
As a general rule, peroxide forming chemicals should not be stored longer than a year.
Certain chemicals are known to form peroxides on exposure to air or light. Peroxide
concentrations may accumulate over long periods of time. The distillation of solvents
contaminated with peroxides may lead to violent explosions as the peroxides become
concentrated during the process. A peroxide present as a contaminating reagent in a
solvent can change the course of a planned reaction.
Keep all stored chemicals, especially flammable liquids, away from heat and direct
sunlight. Peroxide-forming chemicals call for special consideration at all times and
particularly in storage. They should be stored in dark bottles; ultraviolet light and
elevated temperature accelerate peroxide formation.
Peroxide-forming solvents shall be dated when opened and checked for the presence of
peroxides with either wet chemicals or test strips. The checks should be conducted prior
to heating the solvent and after each month of storage. Peroxides may be removed by
99
passing the solvent through an alumina column. The alumina shall not be allowed to dry
out and shall be given to EHS promptly for disposal.
Some peroxide-forming chemicals are listed in Table 6.10. Most typical are ethyl ether,
dioxane, and tetrahydrofuran. They shall not be stored more than six months and shall not
be put into storage without special posting indicating their presence and removal date.
Several acceptable colorimetric tests for peroxides in ethers are available. Contact EHS
for information. If sufficient peroxide is present to form a precipitate, the container and
its contents shall be handled with extreme care. Call EHS to have it removed. Generally,
if you think you should test for the presence of peroxides, then you probably have kept the
material too long and should dispose of it immediately.
A test for peroxides should only be attempted if it is clear that no danger will result from
moving or opening the container. Solids in the liquid or around the cap can indicate
dangerous peroxide buildup.
If old containers of peroxide-forming chemicals are found, do not move them without
consulting EHS. This is especially true if they contain precipitate. If they are to be
moved, handle them only by the bottom of the container and never by the cap or lid, as
friction may cause a violent explosion.
In general, do not attempt to dilute the concentration of peroxides in peroxide-forming
solvents by adding additional solvent. Increasing the total volume may dilute the
peroxide concentration but creates a larger quantity of waste for disposal. The higher
volume of waste may require stabilization because of the presence of the peroxides.

100
TABLE 6.10 LIST OF PEROXIDIZABLE COMPOUNDS
Acetal
Acetaldehyde
Acrylamide
Acrylic Acid
Acrylonitrile
Allyl ethyl ether
Allyl phenyl ether
Allyl vinyl ether
1-Allyloxy-2,3-epoxypropane
Benzyl-1-naphthyl ether
Benzyl butyl ether
Benzyl ethyl ether
Bis(2-ethoxyethyl) ether
Bis(2-methoxyethyl) ether
1,3-Butadiene
1,3-Butadiyne
2-Butanol
Buten-3-yne
Butyl ethyl ether
Butyl formate
Butyl vinyl ether
2-Chloro-1,3-butadiene
1-Chloro-2,2-diethoxyethane
2-Chloroacrynitrile
2-Chloroethyl vinyl ether
Chloroethylene
Chloroprene
Chlorotrifluoroethylene
Cinnamaldehyde
Crotonaldehdye
Cyclohexene
Cyclooctene
Cyclopropyl methyl ether
Decahydronaphthalene
Decalin
Di(2-propynyl)ether
Diacetylene
Diallyl ether
Dibenzyl ether
p-Dibenzyloxybenzene
1,2-Dibenzyoxyethane
Dibutyl ether
1,1-Dichloroethylene
Dicyclopentadiene
1,1-Diethoxyethane
1,2-Diethoxyethane
Diethoxymethane
3,3-Diethoxypropene
Diethyl ether
Diethyl fumarate
Diethylene glycol dimethyl ether
Diethylketene
Digylme
2,3-Dihydrofuran
2,3-Dihydropyran
Diisopropyl ether *
1,1-Dimethoxyethane
1,2-Dimethoxyethane
1,1-Dimethoxypropane
2,2-Dimethoxypropane
3,3-Dimethoxypropene
2,2-Dimethyl-1,3-dioxolane
2,6-Dimethyl-1,4-dioxane
1,3-Dioxane
1,4-Dioxane
1,3-Dioxep-5-ene
1,3-Dioxol-4-en-2-one
Dipropoxymethane
Dipropyl ether
Divinyl acetylene *
Divinyl ether
1,2-Epoxy-3-isopropoxy
propane
1-Ethoxy-2-propyne
2-Ethoxyethanol
2-Ethyl butanal
Ethyl isopropyl ether
Ethyl propenyl ether
Ethyl vinyl ether
2-Ethylacrylaldehyde oxime
Ethylene glycol dimethyl ether
2-Ethylhexanal
2-Ethylhexyl vinyl ether
2-Furaldehyde
Furan
Glyme compounds
4,5-Hexadien-2-yn-1-ol
2,4-Hexadienal
2,5-Hexadiyn-1-ol
2-Hexenal
Indole-2-carboxyaldehyde
Isobutyl vinyl ether
Isobutyraldehyde
Isopropoxypropionitrile
Isopropyl ether *
Isopropyl propyl ether
Isopropyl vinyl ether
2-Isopropylacrylaldehyde
oxime
Isovaleraldehyde
Limonene
1,5-p-Menthadiene
Methoxy-1,3,5,7-cyclo
octatetraene
2-Methoxyethanol
2-Methoxyethyl vinyl ether
Methyl acetylene
Methyl methacrylate
4-Methyl-1,3-dioxane
2-(1-Methylheptyl)-4,6
dinitrophenyl
crotonate
2,3-Methyl-2-methylene
butanal
4-Methyl-2-pentanone
2-Methyltetrahydrofuran
Methyl vinyl ether
2-Penten-4-yn-3-ol
a-Pentylcinnamaldehyde
Potassium * (forms yellow
potassium peroxide on the
surface)
Potassium amide
2-Propanol
Propionaldehyde
2-Propyne-1-thiol
Sodium 5,8,11,14,-
eicosatetraenoate
Sodium amide *
Sodium ethoxyacetylide
Styrene
1,1,2,3-Tetrachloro-1,3,-
butadiene
Tetrafluoroethylene
Tetrahydrofuran
Tetrahydronaphthalene
Tetrahydropyran
Tetralin
Tridecanal
1,3,3-Trimethoxypropene
3,3,5-Trimethyl-2-cyclo-
hexene-1-one
(isophorone)
Vinyl acetate
Vinyl acetylene
Vinyl chloride
Vinyl ethers
Vinyl pyridine
4-Vinylcyclohexene
Vinylidene chloride
*Forms peroxides rapidly upon storage.
NOTE: Compounds with synonyms are listed under all known names.
From Handbook of Reactive Chemicals, L. Bretherick, 1990, and J. Chem Education, Jackson et al., vol. 47,
No. 3 (1970).
101
6.8.5.3 Polynitro Compounds
Many polynitroaromatic compounds are shock-sensitive, as are some aliphatic
compounds containing more than one nitro group. Many of these compounds are sold
and stored with 10 to 20 percent water, which desensitizes their reaction to shock,
although they are still flammable solids.
Storage. Polynitro compounds shall be stored separately from most chemicals and
labeled so they will be easily identified as reactive. They shall not be placed in long-
term storage without special posting indicating their presence and removal date. Long-
term storage without checking for proper water content may allow the compounds to
dehydrate sufficiently to make them highly reactive.
Surplus and waste polynitro compounds shall be given to EHS promptly for proper
disposal or recycling and not left on a shelf to be forgotten.
If old containers of polynitro compounds are found, including picric acid or
dinitrophenyl hydrazine, do not move them without consulting EHS. If they are moved,
handle them only by the bottom of the container and never by the cap or lid, as friction
may cause a violent explosion.
Picric Acid. Dry picric acid is highly explosive and should be brought into the
laboratory only when specifically required. Users should have a thorough understanding
of its hazards. Although not explosive when wetted, picric acid solutions may evaporate
to leave the hazardous solid. Picric acid should be stored away from combustible
materials and should not be kept for extended periods. Old containers of picric acid
shall be handled only by EHS.
Methyl nitronitrosoguanidine. Methyl nitronitrosoguanidine is a carcinogenic agent
that is also shock-sensitive. It is stored in a separate area, preferably locked. Waste
paper, plastic, and glass contaminated with this material shall be given to EHS for
proper disposal.
6.8.5.4 Catalysts
Catalysts such as raney nickel or palladium on carbon shall be filtered from catalytic
hydrogenation reaction mixtures with care. The catalyst has usually become saturated
with hydrogen and will produce flames spontaneously on exposure to air.
6.8.5.5 Calorimeters (Commonly Known as Parr Bombs)
Calorimeters/Parr bombs are pressure reactors designed for handling chemical reactions
and tests at elevated temperatures and pressures. They are intended for the development
102
of new formulations, the study of reaction parameters, or the production of fine
chemicals in small quantities.
Parr bombs shall be handled behind reaction shields. The operator shall wear goggles or
preferably a face shield. The reactor shall never be filled to more than three-fourths of
its available free space. Reactors shall be selected based on the prescribed ratings for
maximum temperature and pressure. The user may not attempt to increase the working
limits by altering the reactor or substituting components not recommended by the
manufacturer. Also, the rating of the burst disc must not exceed the range of the
pressure gage.
The bombs shall be constructed of metals or alloys that provide appropriate corrosion
resistance properties. If necessary, the reactor’s stirrer motor and heater assembly may
need to be explosion-proof.
6.8.5.6 Sodium Azide
Sodium azide is a toxic, highly reactive, heat-sensitive, and potentially shock-sensitive
material. Because it reacts with metals, Teflon or other nonmetal spatulas should be
used with the material. It shall be used with appropriate personal protective gear.
Sodium azide should only be purchased in small quantities, ideally the minimum amount
needed in the laboratory. Storage of solid sodium azide is strongly discouraged. Prepare
stock solutions as soon as the material is delivered to the laboratory.
6.8.5.7 Organometallics
Organometallics are organic compounds comprised of a metal or nonmetal attached
directly to carbon (RM). Examples are Grignard compounds and metallic alkyls such as
triethylaluminum and trimethylindium. Many organometallics are highly toxic or
flammable. Many are also water-reactive and spontaneously combustible in air.
Trialkyltins are the most toxic as a group. Most are highly reactive chemically. Special
firefighting equipment (e.g., dry chemical powder fire extinguisher) may be needed
where organometallics are handled.
6.8.5.8 Hydrides
Hydrides are inorganic compounds composed of hydrogen and another element, often a
metal. Examples include arsine (AsH
3
), phosphine (PH
3
), diborane (B
2
H
6
), germane
(GeH
4
), stibine (SbH
3
), and silane (SiH
4
). The listed hydrides are highly toxic and
flammable. They react violently with water and oxidizing agents and pose a dangerous
fire risk. Phosphine, diborane, and silane are spontaneously flammable in air.
Certain hydride gases, notably arsine and phosphine, are commonly used as dopants in
semiconductor research applications. Arsine is one of the most toxic gases known. It is

103
a potent hemolytic agent (symptoms: red discoloration of the urine and sclera).
Phosphine is extremely toxic to organs of high oxygen flow and demand. Thorough
emergency planning for accidental releases shall be in place when such gases are to be
used in the laboratory. Provision for continuous system monitoring for releases may be
called for.
Exhaust streams of hydride gases shall be treated (e.g., scrubbing or thermal
decomposition) before release. Inform EHS of the treatment procedures to be applied.
6.9 Chemical Waste Management (PSU Safety Policy SY20 Hazardous Waste
Disposal)
The University is required by regulation 25 PA Code Ch. 260 a - 262 a and by Environmental Protection Agency
regulation 40 CFR 260-262 to ensure the proper disposition of these wastes.
Proper handling of reaction byproducts, surplus and waste chemicals, and contaminated materials
is an important part of safety procedures. Each worker is responsible for ensuring that wastes are
handled in a manner that minimizes personal exposure and the potential for environmental
contamination.
The first steps in managing chemical wastes are selecting the least hazardous chemicals for the
task and ordering chemicals only in quantities really needed. Chemicals should not be kept in
laboratories if they will not be needed, especially if they are peroxide-forming chemicals such as
ethyl ether or dioxane, polynitro compounds such as picric acid or dinitrophenyl hydrazine, or
chemicals that are air- or water-reactive.
PSU Safety PolicySY20:
A waste may be designated as a hazardous waste if it meets one of the following criteria:
Acute hazardous waste is a waste which has been found to be fatal in humans in low doses or, in the absence of data on humans, has
been found to have, in laboratory animals:
(A) an oral LD50 of less than 50 mg/kg,
(B) an inhalation LC50 of less than 2 mg/l, or
(C) a dermal LD50 of less than 200 mg/kg.
A waste is hazardous if it contains any of the toxic constituents listed in the regulations.
A waste is hazardous if it exhibits any of the following characteristics:
(A) Ignitability
(C) Reactivity
B) Corrosivity
D) Toxicity
104
POLICY:
The Senior Vice President for Finance and Business establishes and approves the policy and procedure for hazardous waste disposal within the
environment of The Pennsylvania State University. The basis for such policy and procedure shall be recommendations of the University
Hazardous Waste Advisory Board. This Board shall review and recommend revisions to these procedures as appropriate.
Environmental Health and Safety shall be the University agency responsible for implementing and enforcing the established policy and
procedure. This agency shall also be responsible for the coordination of all hazardous waste disposal efforts.
The Directors of Business Services, in conjunction with the individual hazardous waste generators at non-University Park locations, and the
individual hazardous waste generators at University Park, shall be responsible for coordinating the collection of hazardous waste with
Environmental Health and Safety.
The custody and disposition of all chemicals/materials obtained or produced by, for and/or resulting from experiments, research or purchase is the
responsibility of the University employee and/of their organizational unit so pre-occupied. The organization's budget under which such
chemicals/materials are obtained or produced may also be required to fund the analysis of such items which cannot be identified by their proper
or generic name or are improperly labeled. All containers of chemicals/materials must be clearly identified and labeled as to their contents.
UNKNOWNS OR IMPROPERLY LABELED CHEMICALS/MATERIALS WILL NOT BE ACCEPTED FOR DISPOSAL.
Normal hazardous waste disposal costs will be funded through Environmental Health and Safety.
Generators of hazardous waste are responsible to ensure the appropriate storage, labeling, inspection, auditing, documentation, and segregation of
chemicals, and to provide and document training of all personnel involved in the handling of this waste.
The indiscriminate drain-disposal of chemicals/materials is not permitted. Drain disposal of chemical waste materials shall be permitted only with
specific written approval by Environmental Health and Safety.
Departments that generate hazardous chemical wastes shall ensure that a waste reduction program is in effect and that it is being adhered to.
REDUCING HAZARDOUS MATERIALS:
To effect a reduction in the volume of hazardous waste generated at the University, as mandated by the Pennsylvania Department of
Environmental Protection (PA DEP), and the Environmental Protection Agency (EPA), generators of hazardous waste shall minimize the volume
or toxicity of their waste.
Substitutions can be made to eliminate or reduce the amount of hazardous ingredients.
Management practices can greatly reduce unnecessary waste generation. This includes the purchase of only the quantity of material
anticipated to be used and establishing usage parameters for each chemical.
Hazardous materials may be redistributed or returned. Often, surplus chemicals can be redistributed within the University or returned to the
manufacturer. Lists of redistributable chemicals should be circulated among faculty and staff within work units or departments. Such a list
should contain the following information:
chemical name,
amount,
manufacturer,
Purity, as stated on label, and
whether the container is unopened.
EHS maintains a listing of chemicals that are available for redistribution.
Bulking of compatible chemicals. Environmental Health and Safety shall provide guidance in the consolidation of compatible chemicals. A
significant reduction in disposal costs can be achieved in the bulking of these chemicals.
Waste segregation. Mixing wastes can be hazardous; incompatible wastes can react - and explode. Wastes transported to the Chemical
Waste Storage facility must be segregated to avoid these reactions. A further reduction in the costs for waste disposal can be achieved by
reducing packaging time as compatible chemicals can be packed more efficiently. Chemicals should be segregated into the following
categories: flammables, corrosives, poisons, and oxidizers.
Integrate micro-scale techniques into organic and inorganic chemistry laboratory courses and research projects. These techniques can reduce
chemical purchase costs and significantly reduce the quantities of waste chemicals for disposal. Use of micro-scale also reduces student and
faculty exposure to toxic chemicals, carcinogens, flammables and explosives.
RESPONSIBILITIES:
Individuals responsible for laboratories and other areas which handle and store hazardous waste are required to:

105
1. Each room generating chemical waste must designate a location within the room for waste accumulation. This area is referred to
as the "Accumulation Area."
2. Designate an individual who is responsible to oversee the proper storage, labeling and inspection of this Accumulation Area and
who conducts weekly inspections of this area, documenting and maintaining the results of the inspection.
3. Ensure all laboratory personnel involved in chemical waste management are trained and documentation of training records is
maintained.
4. Establish, implement and document an annual review of all hazardous materials to ensure those exceeding safe and practical
usage are properly disposed of.
5. Incorporate waste disposal management practices into all procedures, including laboratory manuals used for instruction.
6. Conduct audits of waste management procedures as established in this policy to ensure compliance and implement the necessary
changes.
Department heads/heads of administrative units are responsible to:
1. Prepare a written program description for compliance with this policy and designate an individual responsible for department-
wide compliance.
2. Maintain a listing of accumulation areas and individuals responsible for oversight.
3. Maintain copies of training documents.
4. Conduct audits of waste management procedures within facilities under their jurisdiction as established in this policy to ensure
compliance and implement the necessary changes.
Deans of Academic Colleges/Heads of Administrative Units are responsible to:
1. Designate a College/Unit-wide individual to oversee program.
2. Conduct audits of waste management procedures established in this policy to ensure compliance and implement the necessary
changes.
PROCEDURES:
Collection and transportation of hazardous waste at University Park:
A laboratory or facility that has hazardous waste for disposal shall fill out a chemical pick up request available at www.ehs.psu.edu.
Environmental Health and Safety personnel will collect and transport the hazardous waste to the Chemical Accumulation Facility.
Procedures for the collection of specially-arranged disposal activities will be established by EHS.
The spill or discharge of any hazardous material must be reported to Environmental Health and Safety at 865-6391 during regular working
hours (8:00 a.m. to 5:00 p.m.). At other times and on weekends, the incident must be reported to University Police. Callers from 862, 863,
and 865 telephones, dial 911; from other numbers, dial 863-1111. Environmental Health and Safety personnel will report to the site of the
incident and provide guidance and direction in proper cleanup procedures, as deemed appropriate. They will provide or recommend
appropriate equipment for the cleanup, and arrange for the proper disposal of the hazardous waste.
Disposal of hazardous waste at non-University Park locations (except Hershey Medical Center):
Other locations that have hazardous waste for disposal will forward a properly completed Chemical Waste Manifest Form to Environmental
Health and Safety.
Environmental Health and Safety will arrange to have the hazardous waste picked up by a commercial vendor.
Penn State complies with U.S. and Pennsylvania Environmental Protection Agency regulations
for disposal of hazardous chemical wastes and with U.S. and Pennsylvania Department of
Transportation regulations for shipment to disposal sites. It is a federal and state offense to

106
dispose of chemicals improperly. EHS is responsible for disposal of chemicals and should be
contacted to arrange the removal of chemical wastes.
6.9.1 Pickups
Chemical wastes are removed by EHS or a contracted vendor at non-University Park
locations. To request a pickup, you must complete a Chemical Waste Pick-up Request
on the EHS website, www.ehs.psu.edu. Chemicals shall not be brought directly to EHS.
Chemical waste containers shall always be labeled with a red tag supplied by EHS with
the complete chemical name. Abbreviations, trade names, or chemical formulas shall
not be permitted. When materials have been added, the amount and concentration of
constituents must be listed on the container tag.
Unused pesticides may be disposed of through the PDA program “CHEMSWEEP” or
through EHS.
6.9.2 Sanitary Sewer Disposal (PSU Safety Policy -
Disposal of Pollutants in University Sanitary Systems (SY40))
It is a violation of both safety and environmental regulations to pour chemicals down the
drain unless they are treated or neutralized and local regulation allows them in the
sanitary sewer system.
The management of hazardous waste is regulated at the federal level by the United States
Environmental Protection Agency (EPA) under the authority of the Resource Conservation and
Recovery Act (RCRA). At the state level, the Pennsylvania Department of Environmental
Resources (PADEP) also regulates the disposal of hazardous waste. These regulations are
contained in 25 PA Code Chapter 262a.
In accordance with these regulations, “the indiscriminate drain-disposal of chemicals/materials”
is prohibited, see PSU policy SY40 Disposal of Pollutants in University Sanitary Systems.
Inappropriate disposal of certain chemicals into the sanitary sewer system may create a variety
of hazards including the following:
Fire and/or explosion hazards within the drain system.
Inadvertent mixing, within the drain system, of incompatible chemicals from different
laboratories.
Corrosion of drainpipes.
Chemical exposure hazards to plumbers.
Escape of volatile, toxic and/or malodorous substances.
Biocidal action on microorganisms that are necessary for the normal and effective
operation of our waste water treatment plant.
107
Addition of unacceptable amounts of toxic substances (e.g., certain heavy metals) to our
sewage sludge and effluent.
6.9.3 Treatment
To reduce chemical hazard, the last step of an experiment may involve chemical
treatment. All methods of treatment require advance approval by EHS. Generally, only
neutralization of inorganic acids and bases is acceptable. Federal regulations and
University policy require investigators to make every effort to minimize the amount and
toxicity of waste removed from University facilities.
6.9.4 Storage
Storage of waste chemicals shall include separation of incompatible materials, as in
"Chemical Storage," Section 6.0. Separate organic and inorganic chemicals.
Waste containers shall be capped at all times and uncapped only for addition of more
waste.
When the amount of flammable liquid present in a laboratory is calculated, flammable
waste volumes shall be included. All stored waste containers must be properly labeled.
6.9.5 Containers
Chemically contaminated laboratory waste is stored in plastic tubs supplied by EHS to
prevent its being inadvertently placed in the trash and to provide secondary containment.
All containers shall be sealed and properly labeled prior to pickup. The container must
be compatible with the chemical waste.
6.9.6 Collection of Sharps
Chemically contaminated sharps such as broken glass, syringes, pipettes, and razors
shall be collected in sturdy, rigid, puncture-resistant containers for proper disposal.
Plastic bags are not suitable for the collection of sharps as they provide those handling
the bags with no protection from needlesticks or cuts.
Keep in mind that the sharps containers will be handled by a number of individuals
before final disposal. It is the responsibility of the sharps users to ensure that the
packaging of the waste does not pose a hazard for providers of disposal service. For
information concerning suppliers of suitable sharps containers, consult EHS.
6.9.7 Mixed Waste

108
Mixed waste is any waste that contains radioactive material and also one or more
hazardous chemical or biological components. Generators shall contact EHS prior to
generating a mixed waste. Special requirements govern the disposal of such waste.
Disposal of some mixed waste is prohibited by law. Generation of mixed waste in a
university laboratory could jeopardize the University's compliance with federal
regulations. Potential mixed-waste generation must be addressed in the planning stage
of any experiment that will create mixed waste.
6.9.8 Waste Minimization
The Environmental Protection Agency's regulations and PSU Safety Policy SY20 for
hazardous waste management place the highest priority on waste minimization. Under
current environmental laws, the University must certify that it has a waste minimization
program in place. In addition, the University must annually report to the government on
efforts it has made to reduce hazardous wastes.
Waste minimization as defined by the EPA means a reduction in both the volume and
physical hazard or toxicity of the material. The benefits of waste minimization include
reduced disposal costs, decreased liability, improved working conditions, and less
impact on the environment at the time of disposal.
The waste minimization policy at the University requires investigators to make every
effort to minimize the volume or the toxicity of their waste. Substitutions can be made
to eliminate or reduce the amount of hazardous components. Wastes can be minimized
by treatment in the lab to yield less toxic or hazardous materials. Experimental
procedures can also be altered to reduce wastes. Finally, improved laboratory
management can result in waste minimization.
It is the responsibility of every investigator who generates waste to incorporate the
principles of waste minimization into experimental design. EHS can help evaluate
procedures for potential waste minimization benefits.
6.10 Nanomaterials*
Exposure standards have not been established for engineered nanoparticles in the United States or
internationally [Safe Nanotechnology 2008.] Until more definitive findings are made regarding the
potential health risks of handling nanomaterials, researchers planning to work with nanomaterials must
implement a combination of engineering controls, work practices, and personal protective equipment to
minimize potential exposures to themselves and others. For a quick guide to the exposure risks and
prudent control measures to be used for common laboratory operations involving nanomaterials, refer to
the table below. It is important to consider if the nanoparticles are in an agglomerated or aggregated
form, functionalized, suspended in liquid, or bound, as these conditions may affect the exposure potential.
6.10.1 Engineering Controls:
109
Use glove bags, glove boxes, fume hoods, or other containment or exhausted enclosures when
there is a potential for aerosolization, such as: handling powders; creating nanoparticles in gas
phase; pouring or mixing liquid media which involves a high degree of agitation. (DO NOT use
horizontal laminar flow hoods (clean benches), as these devices direct the air flow towards the
worker.) Consult with EH&S if engineering controls are not feasible.
Use fume hoods or other local exhaust devices to exhaust tube furnaces and or chemical reaction
vessels.
Perform any maintenance activities, such as repair to equipment used to create nanomaterials or
cleaning/replacement of dust collection systems, in fume hoods or under appropriate local
exhaust.
6.10.2 Work Practices:
Selection of Nanomaterials:
Whenever possible, handle nanomaterials in solutions or attached to substrates to minimize
airborne release.
Consult the Safety Data Sheet (SDS), if available, or other appropriate references prior to using a
chemical or nanomaterial with which you are unfamiliar. Note: Information contained in some
SDSs may not be fully accurate and/or may be more relevant to the properties of the bulk material
rather than the nano-size particles.
Safety Equipment:
Know the location and proper use of emergency equipment, such as safety showers, fire
extinguishers, and fire alarms.
Hygiene:
Do not consume or store food and beverages, or apply cosmetics where chemicals or
nanomaterials are used or stored since this practice increases the likelihood of exposure by
ingestion.
Do not use mouth suction for pipetting or siphoning.
Wash hands frequently to minimize potential chemical or nanoparticle exposure through
ingestion and dermal contact.
Remove gloves when leaving the laboratory, so as not to contaminate doorknobs, or when
handling common use objects such as phones, multiuser computers, etc.
Labeling and Signage:
110
Store in a well-sealed container, preferably one that can be opened with minimal agitation of the
contents.
Label all chemical containers with the identity of the contents (avoid abbreviations/ acronyms);
include term "nano" in descriptor (e.g., "nano-zinc oxide particles" rather than just "zinc oxide."
Hazard warning and chemical concentration information should also be included, if known.
Use cautious judgment when leaving operations unattended: i) Post signs to communicate
appropriate warnings and precautions, ii) Anticipate potential equipment and facility failures, and
iii) Provide appropriate containment for accidental release of hazardous chemicals.
Cleaning:
Wet wipe and or HEPA-vacuum work surfaces regularly.
Transporting:
Use sealed, double-contained container when transporting nanomaterials inside or outside of the
building.
Buddy System:
Communicate with others in the building when working alone in the laboratory; let them know
when you arrive and leave. Avoid working alone in the laboratory when performing high-risk
operations.
6.10.3 Personal Protective Equipment:
Wear gloves, lab coats, safety goggles, long pants, closed-toe shoes, and face shields, as
appropriate dependent on the nature of the materials and procedure.
If work cannot be conducted inside a fume hood or other ventilated enclosure, consult with EHS
814/865-6391 regarding the need for respiratory protection or other alternative controls.
6.10.4 Training:
Ensure that researchers have both general safety training and lab-specific training relevant to the
nanomaterials and associated hazardous chemicals used in the process/experiment.
Lab-specific training can include a review of this safety fact sheet, the relevant Safety Data
Sheets (if available), and the lab's Standard Operating Procedure for the experiment.
6.10.5 Standard Operating Procedures:

111
Prepare a Standard Operating Procedure (SOP) for operations involving nanomaterials. A general
use Standard Operating Procedure for working with nanomaterials is available in below. The SOP
should be tailored to be specific to the proposed experimental procedure.
Consider the hazards of the precursor materials in evaluating the process.
Special consideration should be given to the high reactivity of some nanopowders with regard to
potential fire and explosion. [Pritchard 2004].
6.10.6 Consultation:
Consult with your Principal Investigator prior to procuring or working with nanomaterials. For
additional assistance, contact EHS 814/865-6391.
TABLE 6.11 QUICK GUIDE: EXPOSURE RISKS AND CONTROL MEASURES FOR
COMMON LABORATORY OPERATIONS INVOLVING NANOMATERIALS
Activity types, by Risk of Exposure Primary Control Measures
Low Probability:
Non-destructive handling of solid nanoparticle
composites or nanoparticles permanently
bonded
Disposable nitrile or PVC gloves. Do not reuse
gloves.
Wet cleaning procedures and/or HEPA vacuum
for surfaces/equipment.
Medium / High Probability:
Working w/ nanomaterials in liquid media
during pouring or mixing, or where a high
degree of agitation is involved (e.g.,
sonication)
Handling nanostructured powders*
High speed abrading/grinding nano-composite
materials
Maintenance on equipment used to produce
nanomaterials
Cleaning of dust collection systems used to
capture nanoparticles
Conduct task within a fully enclosed system
(e.g., glovebox), or fume hood
Disposable gloves appropriate for the solvent
in which the particles are suspended. Do not
reuse gloves.
Safety eyewear (+ face shield if splash
potential exists)
Wet cleaning procedures for
surfaces/equipment
High Probability:
Generating nanoparticles in the gas phase or in
aerosol (spill or liquid)
Manipulation of nanoparticles in gas stream
Work in enclosed systems only (e.g.,
glovebox, glovebag, or sealed chamber).

112
* EH&S recognizes that low-density nanomaterials (e.g., carbon-based) become aerosolized by even the slightest
air movement and may not be practical when weighed or handled in laboratory fume hoods.
Consult with EH&S
on alternative sets of controls.
Standard Operating Procedure Template for the
Laboratory Use of Nanomaterials
#1
Contact Information:
Procedure Title
Procedure Author
Date of Creation/Revision
Name of Responsible Person (The PI,
Lab Supervisor, or Autonomous
Researcher)
Work phone:
e-mail:
Location of Work (building/lab #)
#2
Process or Experiment Overview: Description of the process or experiment that the SOP covers. This process may be
described in general terms, such as cleaning and purification of single walled carbon nanotubes.
Frequency:
□ one time □ daily □ weekly □ monthly
□ other:_____________
Duration per Expt:
__________ minutes; or ______hours
#3
Risk Assessment: Identify potential chemical and safety hazards. Special consideration should be given to the high
reactivity of some nanopowders with regard to potential fire and explosion. Consider the hazards of the precursor
materials in evaluating the process.

113
#4
Controls: (Check those that apply to the risks and procedures for the work
that will be done)
#4a Engineering/Ventilation Controls:
Engineering Controls Activity type
□ General laboratory
ventilation
Non-destructive handling of solid nanoparticle composites
or nanoparticles permanently bonded to a substrate
□ Laboratory fume hood; or
□ Exhausted enclosure
Working w/ nanomaterials in liquid media during pouring
or mixing, or where a high degree of agitation is involved
(e.g., sonication)
Handling nanostructured powders*
Maintenance on equipment used to produce nanomaterials
Cleaning of dust collection systems used to capture
nanoparticles
□ Glove box; or
□ Other sealed enclosure
Generating nanoparticles in the gas phase or in aerosol
Manipulation of nanoparticles in gas stream
*If work cannot be conducted with appropriate engineering controls, consult with EHS 814/865-6391.
#4b Personnel Protective Equipment:
□ gloves; indicate type:______________________________
□ safety goggles □ face shield □ lab coats □ other:___________________________
□ appropriate street clothing

114
Respiratory protection is generally not required for lab research, provided the appropriate engineering controls are
employed. For additional guidance on respiratory protection, consult with EHS, 814/865-6391.
#4c Location of Nearest Emergency Safety Equipment:
Item: Location:
Eyewash/Safety Shower
First Aid Kit
Chemical Spill Kit
Fire Extinguisher
Telephone
Fire Alarm Manual Pull Station
#5
Step-By-Step Operating Procedure: Provide a sequential description of work, including details such as chemical
concentrations and when special safety equipment is to be utilized. Clearly label areas where nanomaterials are used.

115
#6
Decontamination:
Upon leaving the nanomaterial work area, remove any personal protective equipment worn and wash hands, forearms, face,
and neck.
After each use (or day), wipe down and or HEPA vacuum the immediate work area and equipment to prevent accumulation
of nanoparticles.
#7
Spill and Accident Procedures:

116
Health Threatening Emergencies (ex. Fire Explosion, Serious Injury, or other Immediate Danger)
1. CALL 911
2. Alert people in the vicinity, activate local alarm systems.
3. Evacuate the area.
4. REMAIN NEARBY TO ADVISE EMERGENCY RESPONDERS.
5. Once personal safety is established, call EHS at 814/865-6391
If Personnel Exposed:
1. Remove exposed/contaminated individual(s) from area, unless unsafe to do so because of (a) medical condition of
victim(s), or (b) potential hazard to rescuer(s).
2. If immediate medical attention is required, notify 911
3. Notify EHS to report the potential exposure by calling 814/865-639).
4. Administer First Aid as appropriate.
5. Flush contamination from eyes/skin using the nearest emergency eyewash /shower for a minimum of 15 minutes. Remove
any contaminated clothing.
6. Take copy of SDS(s) of chemical(s) to hospital with victim.
7. Contact EHS 814/865-639 for follow-up.
Non-Health Threatening Emergencies (ex. spills requiring cleanup assistance)
In the event of a spill or release, which may or has impacted the environment (storm drain, soil, air outside the
building), or spill or release that cannot be cleaned up by local personnel:
1. Notify EHS 814/865-639, during nights and weekends contact 863-1111and EHS staff will be contacted.
2. Provide local notifications to your supervisor.
Small Spills/Local Cleanup:
In the event of a minor spill or release that can be cleaned up by local personnel using readily available equipment (absorbent):
1. Notify personnel in the area and restrict access. Eliminate all sources of ignition.
2. Review the SDS for the spilled material, or use your knowledge of the hazards of the material to determine the appropriate
level of protection.
3. Wearing appropriate Personal Protective Equipment, clean up spill involving nanomaterials using wet methods and/or
HEPA vacuum. DO NOT SWEEP SPILLED OR DRIED NANOMATERIALS. Collect spill cleanup materials in a
tightly closed container. Manage spill cleanup debris as Hazardous Waste.
4. Submit on-line waste pickup request to EHS.
#8
Waste Disposal:
Manage nanoparticle wastes, including contaminated lab debris, as a part of your normal laboratory Hazardous
Waste stream.
Collect and store waste materials in a tightly closed container. Include information describing the base
nanoparticle materials on the waste tag.
#9
Training Requirements:
General Training
□Chemical and Hazardous Waste handling
□Other:________________________

117
Location Where Records Maintained:
Laboratory-specific training (check all that apply):
□Review of SDS for Nanomaterial (if available)
□Review of SDS for other chemicals involved in process/experiment
□Review of this SOP
□Other:_________________________
Location Where Records Maintained:
*Nanomaterial information is courtesy of Stanford University and Larry Gibbs
6.11 CONTROLLED SUBSTANCES IN RESEARCH
6.11.1 Introduction
The University must ensure that research activities that involve controlled substances (also
referred to as drugs or pharmaceuticals) are done in compliance with federal and state laws.
These materials must be stored in a manner that prevents theft or diversion. This information
does not apply to licensed physicians, pharmacists, or veterinarians who are licensed differently
by the DEA.
6.11.2 Licensing
All faculty and staff who wish to work with controlled substances must be licensed by the Drug
Enforcement Administration (DEA, a division of the Department of Justice) to do so. The
University does not have a blanket license to cover purchases of such materials. Information on
applying for a license (DEA Form 225) is available at:
http://www.deadiversion.usdoj.gov/drugreg/reg_apps/225/225_instruct.htm#6
Information on various drug schedules is available at:
http://www.deadiversion.usdoj.gov/drugreg/reg_apps/225/225_instruct.htm#3d
In addition to the application form, faculty and staff will need to provide the DEA with the
following:
The applicant’s social security number.
The applicant’s curriculum vitae.

118
A copy of the research protocol summarizing the procedures to be performed using
controlled substances, including specific information on monitoring drug usage,
inventory control, destruction, security, storage and access to the material.
Names of all persons who will have access to the controlled substances or records.
Note that the State of Pennsylvania does not issue licenses for the use of controlled substances (a
question often asked on the application or by the DEA).
6.11.3 Renewal of License
License holders will be notified by U.S. Mail prior to the expiration of their DEA license. If
continued work with controlled substances is anticipated, licensees are strongly encouraged not to
let their license expire.
6.11.4 Storage
Controlled substances must be stored in a manner that prevents theft or diversion of the material.
Schedule 1 materials (those with a high potential for diversion AND a high potential for abuse)
must be stored in a locked safe; Schedule 2-5 materials must be stored in a substantially
constructed cabinet that is locked at all times and cannot be easily removed from the lab/office.
The location of the safe or cabinet should have limited access during normal working hours and
be secured after hours. The licensee should maintain a list of all persons who are authorized to
have access to controlled substances.
6.11.5 Ordering
Ordering controlled substances requires a DEA license. DEA form 222 is the paper-based form
used to order these materials. It is available at:
https://www.deadiversion.usdoj.gov/webforms/orderFormsRequest.jsp
This form is required to order Schedule I or II controlled substances. Schedule III-V drugs do not
require the use of DEA form 222, but still require a DEA license number.
6.11.6 Recordkeeping
Licensees must maintain detailed inventory records as specified in 21CFR1304.03. All records
must be maintained for at least two years, and five years is advisable. Records should include
purchasing records, which can be an invoice, shipping papers, or a packing slip, and must
contain: the name, address and DEA number of the company where the controlled substance was
purchased, the name of the controlled substance purchased, the size and strength of the controlled
substance purchased, the amount purchased, and the date of receipt (which may be hand-written).
Usage records must include the date that the material is used, the initials of the person dispensing
on behalf of the licensee, the name of the controlled substance, the strength and size of the

119
controlled substance, and the amount dispensed. Records for Schedule I and II drugs must be
kept separately from records for Schedule III – V material.
Complete inventory requirements can be found at 21CFR1304.11. In addition to these specific
requirements, additional general requirements include:
A separate sheet must be used for each compound. The sheet must list the date, amount
of receipt, source of the material, and fate of the material (i.e., destroyed in chemical
reaction, neutralized, disposed of through EHS, etc.). Each licensee must be able to
account for all of any given compound that they are licensed to work with (i.e., licensees
must know the amount they have on hand at any given date). The inventory must be
updated at least annually, and whenever materials are removed from their original
container.
6.11.7 Disposal
Unwanted or outdated controlled substances should be disposed of through Environmental Health
and Safety (EHS). The material will be disposed of through a hazardous waste vendor who is
licensed to provide disposal services. EHS will provide the licensee with written documentation
of the destruction of the material for their records.
6.11.8 Contacting the DEA
PITTSBURGH DISTRICT OFFICE
1781 McKees Rocks Road
Pittsburgh, PA 15136
Diversion and Registration Number: (412) 777-1870
Diversion and Registration Fax: (412) 777-1880
Jurisdiction: Western Pennsylvania (zip codes 150 to 168)
PHILADELPHIA DIVISION
William J. Green Federal Building
600 Arch Street, Room 10224
Philadelphia, PA 19106
Diversion Number: (215) 238-5160
Diversion Fax: (215) 238-5170
Diversion Program Manager - Donetta Spears
Jurisdiction: Delaware and Eastern Pennsylvania

120
7.0 BIOLOGICAL AGENTS
7.1 General
Many laboratory practices and requirements are common to laboratories using chemicals and handling
biological agents. The use of Biological Agents, especially Biohazards, is regulated by University Policy
SY-24. The laboratory procedures described in Section 5.0, "General Safety," and Section 6.0, "Chemical
Hazards," also apply to laboratories using biological agents, and that information will not be repeated in this
part. Most of the information in this section is taken from the book Biosafety in Microbiological and
Biomedical Laboratories (National Research Council, 2009). A copy of the book is available for reference
in EHS.
Biological agent hazards in the laboratory are relatively well defined, especially in the case of conventional
disease-producing agents. Major exceptions to this general observation are oncogenic agents and "slow
virus" infections.
Broadly speaking, two major risk situations can be identified. In the first, known agents are used and are
integral to scientific research or teaching; in the second, potentially harmful biological agents are
endogenous to humans or laboratory animals or to animal tissues or fluids. Examples of these are zoonotic
infections in animal handlers, viral contaminants in human tissues and cell cultures, and lymphocytic or
choriomeningitis-infected animal tumor lines.
Other issues that do not relate specifically to personal safety but should be considered include work with
agents that infect lower animals and plants, especially if an accident could seriously jeopardize the
agricultural sector of the economy. The possibility of cross-contamination by infectious agents in laboratory
animals and media preparation areas can also be a significant problem, especially in common resource
facilities.
7.2 Responsibilities
7.2.1 General
The responsibilities of the department head, principal investigator, and others working with
biological hazards include all those described in Section 2.0 of this document. Additional
responsibilities specific to the handling of biological agents include selecting safety practices based
on awareness of the particular hazard and training all personnel accordingly.
7.2.2 Institutional Biosafety Committee Application
A principal investigator wishing to use a biological agent in research shall complete an Institutional
Biosafety Committee Application form, which may be obtained from the Office for Research
Protections (ORP). ORP determines the biosafety level of the agent, notifies the principal
investigator of the biosafety level, and then the laboratory space is inspected for compliance.
121
7.3 Containment Methods
The term containment is used to describe safe methods for managing infectious agents in the laboratory
environment. The purpose of containment is to reduce exposure of laboratory workers and others to
potentially hazardous agents and to prevent their escape into the outside environment. The three elements of
containment are laboratory practice and technique, safety equipment, and facility design.
7.3.1 Laboratory Practice
The most important element of containment is strict adherence to standard microbiological practices
and techniques. Persons working with infectious agents or infected materials shall be aware of
potential hazards and shall be trained and proficient in the practices and techniques required for
safely handling such material. When standard laboratory practices are not sufficient to control the
hazard associated with a particular agent or laboratory procedure, additional measures may be
needed involving safety equipment and facility design.
7.3.2 Safety Equipment (Primary Barriers)
Safety equipment includes biological safety cabinets, enclosed containers, and other engineering
controls designed to prevent or minimize exposures to hazardous biological materials. The use of
vaccines may in some cases provide an increased level of personal protection.
7.3.2.1 Biological Safety Cabinets
The biological safety cabinet is the principal device used to provide containment of
infectious splashes or aerosols. There are three types of biological safety cabinets: Class I,
Class II, and Class III.
Class I is an open-fronted, negative-pressure, vented cabinet with HEPA-filtered exhaust. It may be
equipped with a front closure and gloves for use as a glove box. The inward face velocity is 75 feet per
minute. Suitable for work with low- or moderate-risk biological agents, it provides protection for personnel
and the environment but not for the product.
Class II cabinets are open-fronted laminar-flow cabinets with an inward face velocity of 75 linear feet per
minute. Class II design resembles that of a fume hood but with HEPA-filtered, recirculated mass airflow
within the workspace. Exhaust air is also filtered. Class II cabinets provide protection for personnel,
product, and the environment. They are designed for work with low- or moderate-risk biological agents.
Class III cabinets provide the highest level of protection. Class III is a totally enclosed glove-box cabinet of
gas-tight construction. The cabinet is maintained under negative air pressure of at least 0.5 inches of water
gauge. Supply air is drawn into the cabinet through HEPA filters, and the exhaust air is filtered by two
122
HEPA filters in series before it is discharged to the outside. Generally, the ventilation system is separate
from the facility's ventilation system. Class III cabinets are suitable for high-risk biological agents.
Biological safety cabinets used to protect workers from hazardous biological agents shall be tested and
certified after installation and before use, any time they are moved, and at least annually. The department
head shall provide annual certification and maintain certification records for the department. Testing shall
meet the criteria in National Sanitation Foundation Standard Number 49. Call EHS for information on the
standard and a list of companies qualified to certify biological safety cabinets.
7.3.2.2 Other Safety Equipment
Other safety equipment includes enclosed containers. An example of an enclosed container
is the safety centrifuge cap, designed to prevent release of aerosols during centrifugation.
Safety equipment also includes personal protective clothing and equipment such as gloves,
coats, gowns, shoe covers, boots, respirators, face masks or shields, and safety glasses or
goggles. This clothing and equipment is generally used in combination with biological
safety cabinets and other devices that contain the agents, animals, or materials being
worked with.
In some situations in which it is impractical to work in biological safety cabinets, personal
protective devices may form the primary barrier between personnel and the infectious
materials. Examples of such situations include certain animal studies, animal necropsy, and
activities relating to maintenance, service, or support of the laboratory facility. Consult
with EHS to develop specific safety procedures.
7.3.3 Secondary Barriers
Secondary barriers protect the environment within the facility and outside the laboratory from
exposure to infectious materials. The design of the facility provides the secondary barrier. The
three facility designs are the basic laboratory, the containment laboratory, and the maximum
containment laboratory.
The Basic Laboratory provides general space where work is done with viable agents that are not
associated with disease in healthy adults; it includes Biosafety Levels 1 and 2 facilities. This
laboratory is also appropriate for work with infectious agents or potentially infectious materials
when the hazard levels are low and laboratory personnel can be adequately protected by standard
laboratory practice. While work is commonly conducted on the open bench, certain operations are
confined to biological safety cabinets. Conventional laboratory designs are adequate.
The Containment Laboratory has special engineering features that enable laboratory workers to
handle hazardous materials without endangering themselves, the community, or the environment.
The containment laboratory is described as a Biohazard Level 3 facility. The features that
distinguish this laboratory from the basic laboratory are the provisions for access control and a

123
specialized ventilation system. In all cases, a controlled access zone separates the laboratory from
areas open to the public.
The Maximum Containment Laboratory has special engineering and containment features that
allow laboratory workers to safely conduct activities involving infectious agents that are extremely
hazardous to humans or capable of causing serious epidemic disease. The maximum containment
laboratory is described as a Biosafety Level 4 facility; it is not applicable to activities at the
University.
7.4 Biosafety Levels
The following guidelines are recommended by the Centers for Disease Control and Prevention and the
National Institutes of Health and have been adopted as required procedure at the University. They are
drawn from the book Biosafety in Microbiological and Biomedical Laboratories (Government Printing
Office ISBN # 978-0-085042-4, Centers for Disease Control and Prevention, and National Institutes of
Health, 2009).
7.4.1 Biosafety Level 1
Biosafety Level 1 is suitable for work involving well-characterized agents not known to
consistently cause disease in immunocompetent adult humans, and present minimal potential
hazard to laboratory personnel and the environment. BSL-1 laboratories are not necessarily
separated from the general traffic patterns in the building. Work is typically conducted on open
bench tops using standard microbiological practices. Special containment equipment or facility
design is not required, but may be used as determined by appropriate risk assessment. Laboratory
personnel must have specific training in the procedures conducted in the laboratory and must be
supervised by a scientist with training in microbiology or a related science.
The following standard practices, safety equipment, and facility requirements apply to BSL-1.
7.4.1.1 Standard Microbiological Practices
1. The laboratory supervisor must enforce the institutional policies that control access to the
laboratory.
2. Persons must wash their hands after working with potentially hazardous materials and before leaving
the laboratory.
3. Eating, drinking, smoking, handling contact lenses, applying cosmetics, and storing food for human
consumption must not be permitted in laboratory areas. Food must be stored outside the laboratory area
in cabinets or refrigerators designated and used for this purpose.
4. Mouth pipetting is prohibited; mechanical pipetting devices must be used.
124
5. Policies for the safe handling of sharps, such as needles, scalpels, pipettes, and broken glassware must
be developed and implemented. Whenever practical, laboratory supervisors should adopt improved
engineering and work practice controls that reduce risk of sharps injuries. Precautions, including those
listed below, must always be taken with sharp items.
These include:
a. Careful management of needles and other sharps are of primary importance. Needles must not be
bent, sheared, broken, recapped, removed from disposable syringes, or otherwise manipulated by
hand before disposal.
b. Used disposable needles and syringes must be carefully placed in conveniently located puncture-
resistant containers used for sharps disposal.
c. Non-disposable sharps must be placed in a hard walled container for transport to a processing area
for decontamination, preferably by autoclaving.
d. Broken glassware must not be handled directly. Instead, it must be removed using a brush and
dustpan, tongs, or forceps. Plastic ware should be substituted for glassware whenever possible.
6. Perform all procedures to minimize the creation of splashes and/or aerosols.
7. Decontaminate work surfaces after completion of work and after any spill or splash of potentially
infectious material with appropriate disinfectant.
8. Decontaminate all cultures, stocks, and other potentially infectious materials before disposal using an
effective method. Depending on where the decontamination will be performed, the following
methods should be used prior to transport.
a. Materials to be decontaminated outside of the immediate laboratory must be placed in a
durable, leak proof container and secured for transport.
b. Materials to be removed from the facility for decontamination must be packed in accordance
with applicable local, state, and federal regulations.
9. A sign incorporating the universal biohazard symbol must be posted at the entrance to the laboratory
when infectious agents are present. The sign may include the name of the agent(s) in use, and the
name and phone number of the laboratory supervisor or other responsible personnel. Agent
information should be posted in accordance with the institutional policy.
10. An effective integrated pest management program is required. (See Appendix G.)
11. The laboratory supervisor must ensure that laboratory personnel receive appropriate training regarding
their duties, the necessary precautions to prevent exposures, and exposure evaluation procedures.
125
Personnel must receive annual updates or additional training when procedural or policy changes
occur. Personal health status may impact an individual’s susceptibility to infection, ability to receive
immunizations or prophylactic interventions. Therefore, all laboratory personnel and particularly
women of childbearing age should be provided with information regarding immune competence and
conditions that may predispose them to infection. Individuals having these conditions should be
encouraged to self-identify to the institution’s healthcare provider for appropriate counseling and
guidance.
7.4.1.2 Special Practices
None required.
7.4.1.3 Safety Equipment (Primary Barriers and Personal Protective Equipment)
1. Special containment devices or equipment, such as BSCs, are not generally required.
2. Protective laboratory coats, gowns, or uniforms are recommended to prevent contamination of
personal clothing.
3. Wear protective eyewear when conducting procedures that have the potential to create splashes of
microorganisms or other hazardous materials. Persons who wear contact lenses in laboratories
should also wear eye protection.
4. Gloves must be worn to protect hands from exposure to hazardous materials. Glove selection should be
based on an appropriate risk assessment. Alternatives to latex gloves should be available. Wash hands
prior to leaving the laboratory. In addition, BSL-1 workers should:
a. Change gloves when contaminated, glove integrity is compromised, or when otherwise necessary.
b. Remove gloves and wash hands when work with hazardous materials has been completed and
before leaving the laboratory.
c. Do not wash or reuse disposable gloves. Dispose of used gloves with other contaminated laboratory
waste. Hand washing protocols must be rigorously followed.
7.4.1.4 Laboratory Facilities (Secondary Barriers)
1. Laboratories should have doors for access control.
2. Laboratories must have a sink for hand washing.
3. The laboratory should be designed so that it can be easily cleaned.
Carpets and rugs in laboratories are not appropriate.
126
4. Laboratory furniture must be capable of supporting anticipated loads and uses. Spaces between
benches, cabinets, and equipment should be accessible for cleaning.
a. Bench tops must be impervious to water and resistant to heat, organic solvents, acids, alkalis,
and other chemicals.
b. Chairs used in laboratory work must be covered with a non-porous material that can be easily
cleaned and decontaminated with appropriate disinfectant.
5. Laboratories windows that open to the exterior should be fitted with screens.
7.4.2 Biosafety Level 2
Biosafety Level 2 builds upon BSL-1. BSL-2 is suitable for work involving agents that pose moderate
hazards to personnel and the environment. It differs from BSL-1 in that: 1) laboratory personnel have
specific training in handling pathogenic agents and are supervised by scientists competent in handling
infectious agents and associated procedures; 2) access to the laboratory is restricted when work is being
conducted; and 3) all procedures in which infectious aerosols or splashes may be created are conducted
in BSCs or other physical containment equipment.
The following standard and special practices, safety equipment, and facility requirements apply to
BSL-2.
7.4.2.1 Standard Microbiological Practices
1. The laboratory supervisor must enforce the institutional policies that control access to the
laboratory.
2. Persons must wash their hands after working with potentially hazardous materials and before leaving
the laboratory.
3. Eating, drinking, smoking, handling contact lenses, applying cosmetics, and storing food for human
consumption must not be permitted in laboratory areas. Food must be stored outside the laboratory
area in cabinets or refrigerators designated and used for this purpose.
4. Mouth pipetting is prohibited; mechanical pipetting devices must be used.
5. Policies for the safe handling of sharps, such as needles, scalpels, pipettes, and broken glassware must
be developed and implemented. Whenever practical, laboratory supervisors should adopt improved
engineering and work practice controls that reduce risk of sharps injuries. Precautions, including
those listed below, must always be taken with sharp items. These include:
a. Careful management of needles and other sharps are of primary importance. Needles must not be
127
bent, sheared, broken, recapped, removed from disposable syringes, or otherwise manipulated by
hand before disposal.
b. Used disposable needles and syringes must be carefully placed in conveniently located
puncture-resistant containers used for sharps disposal.
c. Non-disposable sharps must be placed in a hard walled container for transport to a processing area
for decontamination, preferably by autoclaving.
d. Broken glassware must not be handled directly. Instead, it must be removed using a brush and
dustpan, tongs, or forceps. Plastic ware should be substituted for glassware whenever possible.
6. Perform all procedures to minimize the creation of splashes and/or aerosols.
7. Decontaminate work surfaces after completion of work and after any spill or splash of potentially
infectious material with appropriate disinfectant.
8. Decontaminate all cultures, stocks, and other potentially infectious materials before disposal using
an effective method. Depending on where the decontamination will be performed, the following
methods should be used prior to transport:
a. Materials to be decontaminated outside of the immediate laboratory must be placed in a
durable, leak proof container and secured for transport.
b. Materials to be removed from the facility for decontamination must be packed in accordance
with applicable local, state, and federal regulations.
9. A sign incorporating the universal biohazard symbol must be posted at the entrance to the laboratory
when infectious agents are present. Posted information must include: the laboratory’s biosafety
level, the supervisor’s name (or other responsible personnel), telephone number, and required
procedures for entering and exiting the laboratory. Agent information should be posted in
accordance with the institutional policy.
10. An effective integrated pest management program is required. (See Appendix G.)
11. The laboratory supervisor must ensure that laboratory personnel receive appropriate training regarding
their duties, the necessary precautions to prevent exposures, and exposure evaluation procedures.
Personnel must receive annual updates or additional training when procedural or policy changes occur.
Personal health status may impact an individual’s susceptibility to infection, ability to receive
immunizations or prophylactic interventions. Therefore, all laboratory personnel and particularly women
of childbearing age should be provided with information regarding immune competence and conditions
that may predispose them to infection. Individuals having these conditions should be
encouraged to self-
identify to the institution’s healthcare provider
for appropriate counseling and guidance.
128
7.4.2.2 Special Practices
1. All persons entering the laboratory must be advised of the potential hazards and meet specific entry/exit
requirements.
2. Laboratory personnel must be provided medical surveillance, as appropriate, and offered available
immunizations for agents handled or potentially present in the laboratory.
3. Each institution should consider the need for collection and storage of serum samples from at-risk
personnel.
4. A laboratory-specific biosafety manual must be prepared and adopted as policy. The biosafety
manual must be available and accessible.
5. The laboratory supervisor must ensure that laboratory personnel demonstrate proficiency in
standard and special microbiological practices before working with BSL-2 agents.
6. Potentially infectious materials must be placed in a durable, leak proof container during collection,
handling, processing, storage, or transport within a facility.
7. Laboratory equipment should be routinely decontaminated, as well as, after spills, splashes, or other
potential contamination.
a. Spills involving infectious materials must be contained, decontaminated, and cleaned up by staff
properly trained and equipped to work with infectious material.
b. Equipment must be decontaminated before repair, maintenance, or removal from the laboratory.
8. Incidents that may result in exposure to infectious materials must be immediately evaluated and
treated according to procedures described in the laboratory biosafety manual. All such incidents must
be reported to the laboratory supervisor. Medical evaluation, surveillance, and treatment should be
provided and appropriate records maintained.
9. Animal and plants not associated with the work being performed must not be permitted in the
laboratory.
10. All procedures involving the manipulation of infectious materials that may generate an aerosol
should be conducted within a BSC or other physical containment devices.
7.4.2.3 Safety Equipment (Primary Barriers and Personal Protective Equipment)
1. Properly maintained BSCs, other appropriate personal protective equipment, or other physical
containment devices must be used whenever:
129
a. Procedures with a potential for creating infectious aerosols or splashes are conducted. These may
include pipetting, centrifuging, grinding, blending, shaking, mixing, sonicating, opening containers
of infectious materials, inoculating animals intranasally, and harvesting infected tissues from
animals or eggs.
b. High concentrations or large volumes of infectious agents are used. Such materials may be
centrifuged in the open laboratory using sealed rotor heads or centrifuge safety cups.
2. Protective laboratory coats, gowns, smocks, or uniforms designated for laboratory use must be worn
while working with hazardous materials. Remove protective clothing before leaving for non-laboratory
areas, e.g., cafeteria, library, and administrative offices). Dispose of protective clothing appropriately,
or deposit it for laundering by the institution. It is recommended that laboratory clothing not be taken
home.
3. Eye and face protection (goggles, mask, face shield or other splatter guard) is used for anticipated
splashes or sprays of infectious or other hazardous materials when the microorganisms must be
handled outside the BSC or containment device. Eye and face protection must be disposed of with
other contaminated laboratory waste or decontaminated before reuse. Persons who wear contact
lenses in laboratories should also wear eye protection.
4. Gloves must be worn to protect hands from exposure to hazardous materials. Glove selection should
be based on an appropriate risk assessment. Alternatives to latex gloves should be available. Gloves
must not be worn outside the laboratory. In addition, BSL-2 laboratory workers should:
a. Change gloves when contaminated, glove integrity is compromised, or when otherwise necessary.
b. Remove gloves and wash hands when work with hazardous materials has been completed and
before leaving the laboratory.
c. Do not wash or reuse disposable gloves. Dispose of used gloves with other contaminated
laboratory waste. Hand washing protocols must be rigorously followed.
5. Eye, face and respiratory protection should be used in rooms containing infected animals as determined
by the risk assessment.
7.4.2.4 Laboratory Facilities (Secondary Barriers)
1. Laboratory doors should be self-closing and have locks in accordance with the institutional policies.
2. Laboratories must have a sink for hand washing. The sink may be manually, hands-free, or
automatically operated. It should be located near the exit door.
3. The laboratory should be designed so that it can be easily cleaned and decontaminated. Carpets and
rugs in laboratories are not permitted.

130
4. Laboratory furniture must be capable of supporting anticipated loads and uses. Spaces between
benches, cabinets, and equipment should be accessible for cleaning.
a. Bench tops must be impervious to water and resistant to heat, organic solvents, acids, alkalis,
and other chemicals.
b. Chairs used in laboratory work must be covered with a non-porous material that can be easily
cleaned and decontaminated with appropriate disinfectant.
5. Laboratory windows that open to the exterior are not recommended. However, if a laboratory does
have windows that open to the exterior, they must be fitted with screens.
6. BSCs must be installed so that fluctuations of the room air supply and exhaust do not interfere with
proper operations. BSCs should be located away from doors, windows that can be opened, heavily
traveled laboratory areas, and other possible airflow disruptions.
7. Vacuum lines should be protected with liquid disinfectant traps.
8. An eyewash station must be readily available.
9. There are no specific requirements for ventilation systems. However, planning of new facilities
should consider mechanical ventilation systems that provide an inward flow of air without
recirculation to spaces outside of the laboratory.
10. HEPA filtered exhaust air from a Class II BSC can be safely recirculation back into the laboratory
environment if the cabinet is tested and certified at least annually and operated according to
manufacturer’s recommendations. BSCs can also be connected to the laboratory exhaust system by
either a thimble (canopy) connection or directly exhausted to the outside through a hard connection.
Provisions to assure proper safety cabinet performance and air system operation must be verified.
11. A method for decontaminating all laboratory wastes should be available in the facility (e.g., autoclave,
chemical disinfection, incineration, or other validated decontamination method).
7.4.3 Biosafety Level 3
BL3 work must be conducted in accordance with the facility safeguards, standard microbiological
practices, special practices, and safety equipment described in Biosafety in Microbiological and
Biomedical Laboratories. See Tables 7.5 and 7.6 for lists of biohazardous agents that must be
handled at BL3.
Biosafety Level 3 is applicable to clinical, diagnostic, teaching, research, or production facilities in
which work is done with indigenous or exotic agents that may cause serious or potentially lethal
disease as a result of exposure by inhalation. Laboratory personnel have specific training in
131
handling pathogenic and potentially lethal agents and are supervised by scientists experienced in
working with these agents.
All procedures involving the manipulation of infectious materials are conducted within biological
safety cabinets or other physical containment devices, or by personnel wearing appropriate personal
protective clothing and equipment. The laboratory has special engineering and design features such
as access zones, sealed penetrations, and directional airflow.
Many laboratories may not have all the facility safeguards recommended for Biosafety Level 3. In
these circumstances, acceptable safety may be achieved for routine or repetitive operations (e.g.,
diagnostic procedures involving the propagation of an agent for identification, typing, and
susceptibility testing) in Biosafety Level 2 facilities. However, the recommended standard
microbiological practices, special practices, and safety equipment for Biosafety Level 3 must be
rigorously followed.
7.4.4 Biosafety Level 4
University use of biological agents requiring Biosafety Level 4 containment practices is not
anticipated. Should an investigator wish to conduct research with these agents, a description of the
research shall be submitted to the Institutional Biosafety Committee for review and approval prior
to the initiation of research. Approval is also required by the vice president for research and
graduate studies prior to the initiation of research.
7.5 Biological Spills
A biological spill shall be followed by prompt action to contain and clean up the spill. When a spill occurs,
warn everyone in the area and call for assistance as needed. The degree of risk involved in the spill depends
on the volume of material spilled, the potential concentration of organisms in the material spilled, the hazard
of the organisms involved, the route of infection of the organisms, and the diseases caused by the organisms.
Spills of biological agents can contaminate areas and lead to infection of laboratory workers. Prevention of
exposure is the primary goal in spill containment and cleanup, exactly as in chemical spills. In evaluating
the risks of spill response, generation of aerosols or droplets is a major consideration.
If an accident generates droplets or aerosols in the laboratory room atmosphere, especially if the agent
involved requires containment at Biosafety Level 2 or higher, the room shall be evacuated immediately.
Doors shall be closed and clothing decontaminated. Call EHS, 814-865-6391, to supervise the cleanup. In
general, a 30-minute wait is sufficient for the droplets to settle and aerosols to be reduced by air changes.
Longer waiting periods may be imposed depending on the situation. Laboratory personnel and/or EHS must
exercise judgment as to the need for outside emergency help in evacuation.

132
If a spill of a biological agent requiring containment at Biosafety Level 2 or higher occurs in a public area,
evacuation of the area shall be immediate. The principal investigator shall be responsible for designating
the extent of evacuation until EHS or emergency personnel arrive. Prevention of exposure to hazardous
aerosols is of primary importance.
Anyone cleaning a spill shall wear personal protective equipment (for example, laboratory coat, shoe covers,
gloves, and possible respiratory protection) to prevent exposure to organisms. An air-purifying negative-
pressure respirator with HEPA filter cartridges is generally adequate protection against inhalation of most
biological agents. However, there may be exceptions (e.g., work with tubercle bacilli). Contact EHS for
advice in choosing the correct respiratory protection and for information regarding the requirements that
must be met to wear a respirator.
7.5.1 Sterilization, Disinfection, and Decontamination
An appropriate chemical disinfectant should be chosen that is effective against the organisms
involved in the spill (see Table 7.1). The Environmental Protection Agency recognizes the
following categories of chemical germicides (a germicide is an agent that kills pathogenic
organisms). The information in this section is drawn from Protection of Laboratory Workers from
Infectious Disease Transmitted by Blood, Body Fluids, and Tissue, Tentative Guideline, NCCLS
Document M29-T, Vol. 9, No.1 (National Committee for Clinical Laboratory Standards,
November, 1988).
o Sterilizer or Sterilant: An agent intended to destroy all microorganisms and their spores
on inanimate surfaces.
o Disinfectant: An agent intended to destroy or irreversibly inactivate specific viruses,
bacteria, or pathogenic fungi, but not necessarily their spores, on inanimate surfaces. Most
disinfectants are not effective sterilizers.
o Hospital Disinfectant: An agent shown to be effective against specific organisms such as
Staphylococcus aureus, Salmonella choleraesuis, and Pseudomonas aeruginosa. It may
also be effective against other organisms and some viruses. The labels of all commercially
available hospital disinfectants contain a claim (which must be documented) of
effectiveness for specific agents.
o Antiseptic: A chemical germicide formulated for use on skin or tissue. Antiseptics should
not be used as disinfectants.
Decontamination means a procedure that eliminates or reduces microbial contamination to a safe
level with respect to the transmission of infection. Sterilization and disinfection procedures are
often used for decontamination.
The OSHA Bloodborne Pathogens Standard requires that all equipment and environmental and
working surfaces shall be cleaned and decontaminated after contact with blood or other potentially
infectious materials. The standard also requires decontamination of contaminated work surfaces
133
after completion of procedures, immediately or as soon as feasible after any overt contamination of
surfaces or any spill of potentially infectious material, and at the end of the work shift if the work
surface has become contaminated. All reusable equipment shall be decontaminated immediately or
as soon as feasible upon visible contamination.
It should be emphasized that, for any infectious material, adequate pre-cleaning of surfaces is
important for any disinfection or sterilization procedure. Ten minutes of exposure to a disinfectant
may not be adequate to disinfect objects that have narrow channels or other areas that can harbor
microorganisms. Alcohols, for example, are effective for killing HBV but are not recommended for
this purpose because of their rapid evaporation and the consequent difficulty of maintaining proper
contact times.
Formaldehyde is an OSHA-regulated chemical that is a suspect carcinogen, so its use as a
disinfectant is not recommended.
Iodophors that are registered with the EPA may be effective hard-surface decontaminants when
used per manufacturer's instructions, but iodophors formulated as antiseptics are not suitable for use
as disinfectants.
Chlorine compounds are probably the most widely used disinfectants in the laboratory. An
inexpensive, broad-spectrum disinfectant for use on table tops and similar surfaces can be prepared
by diluting common household bleach (which is a 5.25% sodium hypochlorite solution) to obtain at
least 500 ppm of free available chlorine. A 1:100 dilution of commercial bleach produces a solution
containing 500 ppm free chlorine (approximately 1% bleach solution). Subject to the judgment of
the lab worker, large spills of cultured or concentrated infectious agents should first be flooded or
mixed with a more concentrated disinfectant. In this case, use a 1:10 dilution of commercial bleach,
which produces a solution containing 5000 ppm of free chlorine.
Make the solution fresh each day. Be aware that chlorine compounds may corrode metals,
especially aluminum. While a 10% household bleach solution is a commonly used decontaminant
concentration, it is probably stronger than necessary for ordinary uses. Therefore, the use of higher
concentrations of bleach in chemical fume hoods, and the autoclaving of materials that have been
treated with bleach, should be reserved for significant contamination.
Quaternary ammonium compounds are low-level disinfectants and are not recommended for
spills of human blood, blood products, or other potentially infectious materials.
7.5.2 Decontamination of Spills
The following procedure is recommended for decontaminating spills of agents used at BL2 and for human
blood, body fluids, and other potentially infectious material.
1. Wear gloves and a laboratory coat or gown. Heavyweight, puncture-resistant utility gloves, such
as those used for housecleaning and dishwashing, are recommended.
134
2. Do not handle sharps with the hands. Clean up broken glass or other sharp objects with sheets of
cardboard or other rigid, disposable material. If a broom and dustpan are used, they must be
decontaminated later.
3. Avoid generating aerosols by sweeping.
4. Absorb the spill. Most disinfectants are less effective in the presence of high concentrations of
protein, so absorb the bulk of the liquid before applying disinfectants. Use disposable absorbent
material such as paper towels. After absorption of the liquid, dispose of all contaminated materials
as waste.
5. Clean the spill site of all visible spilled material using an aqueous detergent solution (e.g., any
household detergent). Absorb the bulk of the liquid to prevent dilution of the disinfectant.
6. Disinfect the spill site using an appropriate disinfectant, such as a household bleach solution.
Flood the spill site or wipe it down with disposable towels soaked in the disinfectant.
7. Absorb the disinfectant or allow it to dry.
8. Rinse the spill site with water.
9. Dispose of all contaminated materials properly. Place them in a biohazard bag or other
leakproof, labeled biohazard container for sterilization.
7.5.3 Biological Spill in the Open Laboratory
For a spill in the open laboratory outside a biological safety cabinet, the spill response depends on
the size of the spill and hazard of the material. A minimally hazardous material spilled without
generating appreciable aerosols can be cleaned with a paper towel soaked in a chemical disinfectant.
A spill of a larger volume of hazardous material with aerosol generation requires evacuating the
room, waiting for aerosol reduction, donning personal protective gear (including appropriate
respiratory protection), selecting a disinfectant effective against the organisms involved, and
cleaning as described above. Following cleanup, response personnel shall wash or shower with a
disinfectant soap.
7.5.4 Biological Spill within a Biological Safety Cabinet
A spill that is confined within a biological safety cabinet generally presents little or no hazard to
personnel in the area. However, chemical disinfection procedures are to be initiated at once while
the cabinet continues to operate. The disinfectant shall be one that is active against the organisms of
potential hazard. Flammable liquids, such as ethanol or isopropanol, shall not be used, even if

135
effective, because of the fire hazard of generating dangerous vapor concentrations within the
cabinet that could be ignited by an electrical spark or other source.
Spray or wipe the walls, work surfaces, and equipment with the chosen disinfectant. Allow the
disinfectant to remain on the surface for the appropriate contact time (refer to Table 7.1 B for
recommended contact times). Minimize the generation of aerosols and use sufficient disinfectant to
ensure that drain pans and catch basins below the work surface contain disinfectant. The front
exhaust shall also be wiped and the disinfectant drained into a container.
7.5.5 Biological Spill in a Centrifuge or Other Equipment
A biological spill in a centrifuge has the potential for producing large volumes of aerosols. On
becoming aware that a spill may have occurred within a centrifuge or other piece of equipment, turn
off the equipment, warn others in the area, notify the principal investigator, allow aerosols to settle,
and decontaminate following the principles described above.
7.5.6 Biological Spill on a Person
If a biological material is spilled on a person, emergency response is based on the hazard of the
biological agent spilled, the amount of material spilled, and whether significant aerosols were
generated. If aerosol formation is believed to have been associated with the spill, a contaminated
person shall leave the contaminated area immediately. If possible, he or she should go to another
laboratory area so that hallways and other public areas do not become contaminated.
Contaminated clothing is removed and placed in red or orange biohazard bags for disinfecting.
Contaminated skin shall be flushed with water and thoroughly washed with a disinfectant soap.
Showering may be appropriate, depending on the extent of the spill.
7.6 Human Blood, Blood Products, and Other Potentially Infectious Materials
Penn State University has chosen to comply with the OSHA Occupational Exposure to Bloodborne
Pathogens Standard found in Title 29, Code of Federal Regulations, and Part 1910.1030. The information in
this section summarizes the requirements of the standard. Additional information on the Bloodborne
Pathogen Program is available from EHS.
The standard applies to employees who have occupational exposure to
Blood (human blood, human blood components, and products made from human blood)
The following human body fluids:
136
semen
vaginal secretions
cerebrospinal fluid
synovial fluid
pleural fluid
pericardial fluid
peritoneal fluid
amniotic fluid
saliva (in dental procedures)
any body fluid that is visibly contaminated with blood
all body fluids in situations where it is difficult
or impossible to differentiate between body fluids
Any unfixed tissue or organ (other than intact skin) from a human, living or dead.
HIV-containing cell, tissue, or organ cultures; HIV- or HBV-containing culture medium or other
solutions; and blood, organs, or other tissues from experimental animals infected with HIV or HBV.
Occupational exposure means reasonably anticipated skin, eye, mucous membrane, or parenteral contact
with blood or other potentially infectious materials that may result from the performance of an employee's
duties.
7.6.1 Exposure Control Plan
The standard requires a written Exposure Control Plan, the University's plan to identify and control
exposures to blood and other potentially infectious materials. The Exposure Control Plan defines the scope
of the program, provides definitions, explains the exposure determination process, lists methods of
compliance with the standard, and provides information on post-exposure evaluation and training.
The OSHA standard requires review and updating of the Exposure Control Plan at least annually as well as
whenever necessary to reflect new or modified tasks and procedures that affect occupational exposure and
new or revised employee positions with occupational exposure.
7.6.2 Unit Specific Plan
Principal investigators and supervisors in departments with laboratories are required to complete Unit
Specific Plans if they use human blood, blood products, and other potentially infectious materials. The Unit
Specific Plan augments, but is not a substitute for, an Exposure Control Plan. Both plans are required for
laboratory facilities, although the Exposure Control Plan does not require approval by the Institutional
Biosafety Committee.
7.6.3 HIV and HBV Research Laboratories and Production Facilities
137
In the OSHA standard, a research laboratory is defined as a laboratory using research-laboratory-scale
amounts of HIV or HBV. Research laboratories may use high concentrations of HIV/HBV but not in the
volume found in production facilities. A laboratory working with human blood or other potentially
infectious materials containing clinical levels of HIV or HBV is not considered an HIV/HBV research
laboratory. Although compliance with most of the standard is required, most laboratories will not be subject
to the additional requirements for HIV/HBV research laboratories.
A production facility is defined as a facility engaged in industrial-scale, large-volume or high-concentration
production of HIV or HBV.
HIV and HBV research laboratories and production facilities are subject to more stringent regulations.
Consult the OSHA standard or contact OHS if you are unsure whether your laboratories qualify and for
information about additional requirements for these facilities.
7.6.4 Universal Precautions
The practice of universal precautions is an approach to infection control in which all human blood and
other potentially infectious materials are treated as if known to be infectious for HIV, HBV, and other
bloodborne pathogens.
Universal precautions shall be observed to prevent contact with blood or other potentially infectious
materials. When it is difficult or impossible to differentiate between fluid types, universal precautions shall
be observed.
7.6.5 Engineering and Work Practice Controls
Engineering controls are controls that isolate or remove the bloodborne pathogens hazard from the
workplace. Examples are sharps containers and self-sheathing needles. Work practice controls are
controls that reduce the likelihood of exposure by altering the manner in which a task is performed. If a
likelihood of occupational exposure remains even when engineering and work practice controls are in place,
then personal protective clothing shall also be used. Human blood, blood products, and other potentially
infectious materials shall be used with Biosafety Level 2 work practices, containment, and facilities.
a. Handwashing. The department shall provide readily accessible handwashing facilities or,
if this is not feasible, an appropriate antiseptic hand cleanser and clean cloth or paper
towels. In any case, employees shall wash hands with soap and running water as soon as
feasible after possible exposure.
The department head shall ensure that employees wash hands immediately or as soon as
feasible after removing gloves or other personal protective equipment and that employees
wash hands and any other skin with soap and water, or flush mucous membranes with
water, immediately or as soon as feasible following contact of such body areas with
potentially infectious materials.
138
b. Needles and Sharps. Contaminated needles and other contaminated sharps shall not be
bent, recapped, or removed except as noted below. Shearing or breaking contaminated
needles is prohibited.
Contaminated needles and sharps shall be recapped or removed only when no alternative is
feasible or when required by a specific medical procedure. Any recapping or removal must
be accomplished using a mechanical device or a one-handed technique. The recapping or
removal of contaminated sharps is actively discouraged under any circumstances because
of the high potential risk of injection.
Immediately after use, contaminated reusable sharps shall be placed in sharps containers
that are puncture-resistant, labeled or color coded, and leakproof.
c. Eating, Drinking, Smoking, etc. Eating, drinking, smoking, applying cosmetics or lip
balm, and handling contact lenses are prohibited in work areas with a reasonable likelihood
of occupational exposure. Food and drink shall not be kept in refrigerators, freezers,
shelves, or cabinets or on countertops or benchtops where blood or other potentially
infectious materials are present.
d. Splashing, Spraying, Spattering. All procedures involving blood or other potentially
infectious materials shall be performed so as to minimize splashing, spraying, spattering,
and generation of droplets.
e. Mouth Pipetting. Mouth pipetting of blood or other potentially infectious materials is
prohibited.
f. Specimen Containers. The standard includes detailed requirements for specimen
containers. In general, specimens of blood or other potentially infectious materials shall be
placed in a container that prevents leakage during collection, handling, processing, storage,
transport, or shipping. Secondary containers are used when the outside of the primary
container may be contaminated and when puncture of the primary container is possible.
Storage, transport, or shipping containers are closed and labeled; the label should include
the biohazard symbol. Color-coded containers should be red or orange.
g. Potentially Contaminated Equipment. Any equipment to be serviced or shipped that
may be contaminated shall be examined prior to servicing or shipping and decontaminated
as necessary. If decontamination is not feasible, then the equipment shall be clearly labeled
as to which portions remain contaminated. The department is obligated to communicate
this information clearly to employees, service personnel, and manufacturers as appropriate.
h. Other Engineering Controls. Other engineering controls include biological safety
cabinets (e.g., tissue culture hoods) and chemical fume hoods. Engineering controls shall
be examined and maintained on a regular schedule.
139
Chemical fume hoods used for containment of potentially infectious material are inspected
by EHS according to a regular schedule. The Office of Physical Plant (OPP) inspects and
maintains chemical fume hood fan and duct systems.
Biological safety cabinets should be certified on installation, whenever they are moved, and
annually, especially if they are used with pathogens. Certification shall be in accordance
with National Sanitation Foundation Standard Number 49 or manufacturers specifications.
7.6.6 Personal Protective Equipment
a. Responsibility. The department head shall provide or ensure provision of appropriate
personal protective equipment to each employee who is subject to occupational exposure to
human blood or potentially infectious material. The equipment is provided at no cost to the
employee. Appropriate equipment does not permit blood or other potentially infectious
materials to pass through to or reach the street clothes. Examples of such equipment include
gloves, gowns, laboratory coats, head and foot coverings, face shields, masks, eye
protection, resuscitation bags, pocket masks, and other ventilation devices.
The department head shall either directly or by delegation ensure that each employee uses
personal protective equipment when warranted.
b. Availability. Protective equipment in appropriate sizes shall be available in the work area
or issued to employees. Hypoallergenic gloves or similar alternatives shall be readily
available to those allergic to the gloves normally provided.
c. Maintenance. The department head shall ensure that personal protective equipment be
cleaned, laundered, or disposed of at no cost to the employee. Personal protective
equipment shall be repaired or replaced as needed to maintain its effectiveness.
d. Gloves. Gloves shall be worn when it is reasonably anticipated that employees may have
hand contact with blood, other potentially infectious materials, mucous membranes, or
nonintact skin as well as when employees perform vascular access procedures and handle
or touch contaminated items or surfaces.
Disposable gloves shall be replaced as soon as practical when contaminated or when torn,
punctured, or otherwise compromised in their ability to function as a barrier.
Utility gloves (nondisposable gloves) may be decontaminated for reuse provided the
integrity of the glove is not compromised. They must be discarded if they are cracked,
peeling, torn, or punctured or exhibit other signs of deterioration.
Gloves shall be removed prior to leaving the work area.
140
For specific regulations related to phlebotomy, see the OSHA standard or contact EHS.
e. Masks, Eye Protection, and Face Shields. Masks in combination with eye protection
devices (such as goggles or glasses with solid side shields) or chin-length face shields shall
be worn whenever splashes, spray, spatter, or droplets of blood or other potentially
infectious material may be generated and eye, nose, or mouth contamination can be
reasonably anticipated.
f. Gowns, Aprons, and Other Protective Body Clothing. Appropriate protective body
clothing shall be worn in occupational exposure situations. When gross contamination can
be anticipated, surgical caps or hoods and shoe covers should be worn. Contaminated
clothing shall not be worn outside the work area.
g. Implementation for Personal Protective Clothing and Equipment. Appropriate
personal protective equipment shall be worn by workers occupationally exposed to blood or
other potentially infectious materials unless, under rare and extraordinary circumstances,
the use of such equipment would prevent the delivery of health care or public safety
services or would pose an increased hazard to the safety of the worker or a coworker.
Should such a situation occur, the circumstances shall be investigated and documented to
prevent future occurrences.
7.6.7 Housekeeping
a. Responsibility. The department head is responsible for ensuring that the work area shall
be maintained in a clean and sanitary condition. A written schedule for cleaning and
method of decontamination is required.
b. Cleaning. All equipment and environmental and working surfaces shall be cleaned and
decontaminated with an appropriate disinfectant after contact with blood or other
potentially infectious material. Contaminated work surfaces shall be decontaminated after
completion of procedures, immediately or as soon as feasible after any contamination of
surfaces or any spill of blood or other potentially infectious materials, and at the end of the
work shift if the surface may have become contaminated since the last cleaning.
Protective coverings such as plastic-backed absorbent paper shall be removed and replaced
as soon as feasible when they become overtly contaminated or at the end of the work shift
if they may have become contaminated during the shift.
All bins, pails, cans, and similar receptacles intended for reuse that have a reasonable
likelihood of becoming contaminated shall be inspected and decontaminated on a regularly
scheduled basis. They shall be cleaned or decontaminated immediately or as soon as
feasible if there is visible contamination.
141
Chemical disinfectants are listed in Tables 7.1 (A) and (B) with their usage parameters,
applications, and the organisms for which they are effective. Any of the disinfectants listed
are effective for bloodborne pathogens. Purchased disinfectants are recommended if their
parameters meet those described. If other disinfectant materials are used, they are to be
listed in the department-specific information.
c. Broken Glassware. Broken glassware shall not be picked up directly with the hands. It
shall be cleaned up using mechanical means, such as a brush or dustpan, vacuum cleaner,
tongs, or forceps.
7.6.8 Waste Disposal
a. Contaminated Sharps. Contaminated sharps shall be discarded immediately or as soon as
feasible in containers that are closable, puncture-resistant, leakproof, and labeled or color-
coded. Sharps containers shall be easily accessible to employees and located close to the
immediate area where sharps will be used. They shall be maintained upright throughout
use, be replaced routinely, and not be allowed to overfill.
Before sharps containers are removed from the work area, they shall be closed securely. If
leakage is possible, a closable, sturdy, leakproof, and labeled or color-coded secondary
container shall be used.
b. Other Biohazardous Wastes. Waste containers that contain blood or other potentially
infectious material shall be sealable, large enough to contain all intended contents,
leakproof, labeled and/or color-coded, and closed securely prior to removal. If the primary
waste container is contaminated on the outside, a closable, sturdy, leakproof, and labeled or
color-coded secondary container shall be used and shall also be closed prior to removal.
All wastes shall be appropriately decontaminated before disposal (e.g., wastes should be
autoclaved). Once decontaminated, the waste shall be overbagged.
7.6.9 Laundry
a. Instructions. Contaminated laundry shall be handled as little as possible and with a
minimum of agitation. It shall be placed in bags or containers at the point of use. It shall
not be sorted or rinsed at the location of use. The bags or containers shall be labeled with
the biohazard symbol or color-coded (red or orange). The bag or container shall be
constructed to prevent soak-through or leakage.
The department head shall ensure that employees who handle contaminated laundry shall
wear protective gloves and other appropriate personal protective equipment.
Whenever possible, contaminated laundry is disinfected or sterilized prior to submission to
the laundry service for cleaning. Contaminated sharps shall never be included with
142
laundry. Contaminated laundry is never washed with an individual's personal belongings or
sent to a laundry service without notice of the hazards.
7.6.10 Hepatitis B Vaccination and Postexposure Evaluation and Follow-up
a. Responsibility. EHS is responsible for making the hepatitis B vaccine and vaccination
series available to all employees who have occupational exposure and for making post-
exposure evaluation and follow-up available to all employees who have sustained an
exposure incident. All laboratory tests shall be conducted by an accredited laboratory at no
cost to the employee.
b. Hepatitis B Vaccination. Hepatitis B vaccination shall be made available after the
employee has received the training required in this document section and within 10
working days of initial assignment to all employees who have occupational exposure,
unless the employees have previously received the complete hepatitis B vaccination series,
antibody testing has revealed that the employee is immune, or the vaccine is
contraindicated for medical reasons. Participation by the employee in a prescreening
program shall not be a prerequisite for receiving hepatitis B vaccination.
Vaccinations are provided through the Occupational Medicine Division of University
Health Services.
HBV vaccination programs have been established on each campus. Contact EHS for
specific instructions.
c. Postexposure Evaluation and Follow-up. Following a report of an exposure incident, the
department head shall ensure that a confidential medical evaluation and follow-up are made
available to the exposed employee.
d. Medical Records. Occupational Medicine shall ensure that an accurate record is
maintained for each employee with occupational exposure. Employee medical records
shall be kept confidential and shall not be disclosed or reported to any person without the
employee's express written consent except as required by the OSHA standard and by law.
Employee medical records shall be maintained for at least the duration of employment plus
30 years.
7.6.11 Communication of Hazard to Employees: Labels
Warning labels are required on containers of biohazardous waste (unless the waste is placed in red
or orange bags), refrigerators and freezers containing blood or other potentially infectious material,
143
and other containers used to store, transport, or ship blood or other potentially infectious materials.
The labels shall include the biohazard symbol and the word "biohazard."
7.6.12 Communication of Hazard to Employees: Information and Training
a. Responsibility. The principal investigator is responsible for ensuring that all employees
with occupational exposure participate in a training program, which must be provided
during working hours at no cost to the employee.
b. Schedule. Training shall be provided at the time of initial assignment to tasks where
occupational exposure may take place and at least annually thereafter. The annual training
shall be provided within one year of previous training.
c. Additional Training. The principal investigator shall ensure that employees receive
additional training when changes, such as modifications of tasks and procedures or
institution of new tasks or procedures, affect the employee's occupational exposure.
d. Language, Literacy, and Educational Level. Training shall consist of material which,
insofar as possible, shall be appropriate in content and vocabulary to the educational level,
literacy, and language of employees.
e. Content. Training may be provided through a combination of videotapes, handouts, pre-
and posttests, and personal presentations. Each training session shall include an
opportunity for employees to ask questions. Minimum training program requirements have
been provided to department heads.
f. Records. EHS shall maintain training records which shall be kept in the department for
three years from the date on which the training occurred. Training records shall be
provided to employees and to employee representatives on request for examination and
copying.
7.7 Recombinant DNA Activities
Recombinant DNA research shall comply with the National Institutes of Health's "Guidelines for Research
Involving Recombinant DNA Molecules" and by University Policy SY-24. The Institutional Biosafety
Committee is responsible for implementing the guidelines and overseeing recombinant DNA research.
Principal investigators intending to use recombinant DNA molecules shall notify the University Biosafety
Committee by contacting the Office for Research Protections (ORP) for information and appropriate
registration forms. The principal investigator shall prepare registration documents according to the nature of
the research. Principal investigators working with or planning to work with recombinant DNA molecules
shall contact ORP for further information.

144
In general, the containment practices to be used for recombinant DNA research shall follow those described
for Biosafety Levels 1, 2, and 3 in the CDC-NIH Biosafety in Microbiological and Biomedical Laboratories.
However, the NIH Recombinant DNA guidelines take precedence.
7.8 Animal Studies
Animal studies involving the use of hazardous biological agents represent special problems in containment.
Policies and operational practices governing the use of animal containment facilities are under the direction
of the Institutional Animal Care and Use Committee (IACUC) and the Animal Resource Program (ARP).
In general, practices for Animal Biosafety Levels 1, 2, and 3 presented in the CDC-NIH manual, Biosafety
in Microbiological and Biomedical Laboratories, are followed.
Experiments involving the use of infectious biological agents in animals are generally conducted in
containment facilities. Research in laboratory facilities shall be reviewed and approved, prior to the
initiation of work, by the Institutional Biosafety Committee in conjunction with the Animal Care and Use
Committee.
7.9 Infectious Waste Management (PSU Safety Policy SY29 Infectious Waste Disposal)
Infectious waste materials shall be treated properly to eliminate the potential hazard that these wastes pose
to human health and the environment. Management of Infectious Waste is governed by University Policy
SY-29.
7.9.1 Separation and Packaging of Infectious Waste
Infectious wastes shall be separated from general, noninfectious waste materials and from wastes
containing radioactive, carcinogenic, or toxic materials. Some wastes may contain multiple hazards.
These shall be handled such that priority is given to the greatest hazard present. Contact EHS (814-
865-6391) for information on handling mixed biological/radioactive waste. In general, it is easier to
inactivate the biohazard and then deal with the material as radioactive or hazardous waste.
Disposable infectious materials shall be placed in red or orange plastic bags. The bags shall be
seamless, tear-resistant, and autoclavable. Single bags shall have a minimum thickness of 3.0 mils
and double bags, 1.5 to 2.0 mils. Bags shall be closed by folding or tying when full, at the end of
the day, or before transporting.
To minimize formation of aerosols, infectious wastes shall not be compacted prior to
decontamination.
7.9.2 Storage and Transport of Infectious Waste

145
Infectious wastes that are removed from a laboratory or stored temporarily shall be closed and
double- bagged or placed inside a covered, unbreakable outer container.
7.9.3 Infectious Waste Treatment
Infectious wastes are generally rendered noninfectious by autoclaving. The janitorial/custodial staff
has been instructed not to touch or remove red or orange bags. Liquid wastes may also be rendered
noninfectious by adding a sufficient amount of household bleach (5.25% Sodium Hypochlorite) to
account for 10% of the final volume. (i.e., 100 ml of bleach to 1000 ml of liquid waste and incubate
for at least 1 hour). Sterilized liquid wastes may be discarded to the sewer.
Infectious waste that is also radioactive must be autoclaved and then placed in radioactive waste
containers for disposal through EHS.
Sharps that are radioactive must be placed into sharps containers and then placed in radioactive
waste containers for disposal through EHS.
As described under procedures for Biosafety Levels 2 and 3 and procedures for human blood and
related materials, syringes and needles shall be handled with extreme caution to avoid
autoinoculation and the generation of aerosols. Needles shall not be bent, sheared, replaced in the
sheath or guard, or removed from the syringe following use. The needle and syringe shall be
promptly placed in a puncture-resistant container and decontaminated, preferably by autoclaving.
Needles may be rendered unusable following sterilization. Grinding, compaction, or clipping in a
destruction device are acceptable techniques for destroying sterile needles.
All human blood, blood products, nonfixed human tissues, and other potentially infectious materials
are considered infectious and shall be disinfected by steam sterilization.
Infectious wastes, including cultures and stocks of etiologic agents, shall be made noninfectious by
steam sterilization or chemical inactivation.
Animal carcasses, bedding, and wastes are handled by the Animal Resource Program (ARP).
7.9.3.1 Steam Sterilization
Most infectious wastes are sterilized, based on the type of waste, load volume, packaging
material, and load configuration. It is recommended that the efficacy of the autoclave be
monitored using Bacillus stearothermophilis. The frequency of monitoring depends on the
hazard of the organism being used and the frequency of waste sterilization.
Infectious wastes that also contain volatile chemicals should be autoclaved only if a
chemical (hydrophobic) filter is on line. EHS shall be contacted before steam sterilizing
wastes containing carcinogens or radionuclides.
146
7.9.3.2 Chemical Disinfection
Chemical treatment is usually a disinfection rather than sterilization. Thus it is usually
intended as a temporary measure to control infectious wastes until sterilization can treat the
hazard. Disinfection may be used as final treatment on a case-by-case basis following a
petition by the principal investigator and approval by EHS.
Section 7.5.1 and Tables 7.1 (A) and (B) summarize information on practical disinfectants.
Commercially available chlorine bleach is 5.25 percent chlorine (52,500 ppm).
Note that bleach will react with water to form hypochlorous acid (HOCl), which will decompose to
chlorine (Cl
2
) and hydrogen chloride (HCl). Special care should be taken when autoclaving
hypochlorite solutions because the procedure can generate chlorine gas, which will corrode steel. To
avoid evolution of chlorine, the hypochlorite solution should be neutralized with sodium thiosulfate
prior to autoclaving.
7.9.3.3 Mixed Waste
Infectious waste that is mixed with radioactive waste or chemical waste requires special
handling. Liquid infectious waste that contains radioactive material must be rendered
biologically inactive before the Radiation Protection Office will accept it. This can be
done by autoclaving or adding sufficient household bleach (5.25% sodium hypochlorite)
to make up 10% of the total volume (i.e., 100 ml of bleach for each liter of liquid waste).
Solid infectious waste containing radioactive material must be autoclaved prior to
disposal.

147
TABLE 7.1 (A) SUMMARY OF PRACTICAL DISINFECTANTS
CATEGORY
DILUTION
CONTACT TIME IN
MINUTES
IRRITANT TYPE
LIPOVIRUS
BROAD
SPECTRUM
SKIN
EYE
RESPIR.
Quaternaryam
monia cpds. (l)
0.1-2.0% 10 Not
effective
Yes Yes No
Phenolic cpds.
(l)
1.0-5.0% 10 Not
effective
Yes Yes No
Chlorine cpds.
(l)
**500 ppm 10 30 Yes Yes Yes
Iodophor cpds.
(l)
25-1,600
ppm
10 30 Yes Yes No
Ethyl alcohol
(l)
70-85% 10 Not
effective
No Yes No
Isopropyl
alcohol (l)
70-85% 10 Not
effective
No Yes No
Formal-
dehyde (l)
0.2-8.0% 10 30 Yes Yes No
Glutaral-
dehyde (l)
2% 10 30 Yes Yes No
Ethylene oxide
(g)
8-23 g/ft
3
60 60 Yes Yes Yes
Parafor-
maldehyde (g)
0.3 g/ft
3
60 60 Yes Yes Yes
l = liquid; g = gas
**Commercially available chlorine bleach is 5.25% chlorine (52,200 ppm). A dilution of 1 to 100 will
yield a 525 ppm solution, which is suitable for disinfecting purposes.

148
Source: Laboratory Safety Monograph, U.S. Department of Health, Education, and Welfare, Public
Health Service, and National Institutes of Health, 1979.
TABLE 7.1 (B) SUMMARY OF PRACTICAL DISINFECTANTS
DISINFECTANTS
USE PARAMETERS
Concentration of active
ingredient
Temperature
o
C
Relative Humidity
%
Contact time
(minutes)
Ethylene oxide 400-800 mg/l 35-60 30-60 105-240
Paraformaldehyde (gas) 0.3 g/ft
3
>23 >60 60-180
Quaternary ammonium
compounds
0.2-1% 10-30
Phenolic compounds 0.2-3% 10-30
Chlorine compounds 0.01-5% 10-30
Iodophor compounds 0.47% 10-30
Alcohol (ethyl or isopropyl) 70-85% 10-30
Formaldehyde (liquid) 4-8% 10-30
Glutaraldehyde 2% 10-600

149
DISINFECTANTS
EFFECTIVE AGAINST:
Vegetative
bacteria
Bacterial
Spores
Lipo
viruses
Hydrophilic
viruses
Tubercle
bacilli
HIV HBV
Ethylene oxide + + + + + + +
Paraformaldehyde (gas) + + + + + + +
Quaternary ammonium
compounds
+ + +
Phenolic compounds + + ± + + ±
Chlorine compounds + ± + + + + +
Iodophor compounds + + ± + + ±
Alcohol (ethyl or isopropyl) + + ± + ±
Formaldehyde (liquid) + ± + + + + +
Glutaraldehyde + + + + + + +
+ denotes very positive response; ± denotes less positive response; a blank denotes a negative response
or not applicable.
Adapted from Biosafety in the Laboratory: Prudent Practices for the Handling and Disposal of Infectious
Materials, National Research Council, 1989.

150
TABLE 7.2: AGENT SUMMARY STATEMENTS AVAILABLE
The following agent summary statements are available from EHS. Source: Biosafety in Microbiological
and Biomedical Laboratories, HHS Publication No. (CDC) 93-8395, CDC/NIH, 4th edition, May 1999.
Nematode Parasites of Humans
Protozoal Parasites of Humans
Trematode Parasites of Humans
Cestode Parasites of Humans
Fungal Agents
Blastomyces dermatitidis
Coccidioides immitis
Cryptococcus neoformans
Histoplasma capsulatum
Sporothrix schenckii
Pathogenic Members of the Genera Epidermophyton, Microsporum and Trichophyton
Miscellaneous Molds
Bacterial Agents
Bacillus anthracis
Bordetella pertussis
Brucella (B. abortus, B. canis, B. melitensis, B. suis)
Campylobacter (C. jejuni/C. coli, C. fetus subsp. fetus)
Chlamydia psittaci, C. pneumoniae, C. trachomatis
Clostridium botulinum
Clostridium tetani
Corynebacterium diphtheria
Francisella tularensis
Leptospira interrogans - all serovars
Legionella pneumophila; other Legionella-like agents
Mycobacterium leprae
Mycobacterium spp. other than M. tuberculosis, M. bovis or M. leprae
Mycobacterium tuberculosis, M. bovis
Neisseria gonorrhoeae
Neisseria meningitidis
Pseudomonas pseudomallei
Salmonella - all serotypes except typhi
Salmonella typhi
Shigella spp.
Treponema pallidum
Vibrionic enteritis (Vibrio cholerae, V. parahaemolyticus)
Yersinia pestis
Rickettsial Agents
Coxiella burnetii
151
Rickettsia prowazekii, Rickettsia typhi (R. mooseri), Rickettsia tsutsugamushi, Rickettsia canada,
and Spotted Fever Group agents of human disease; Rickettsia rickettsii, Rickettsia conorii,
Rickettsia akari, Rickettsia australis, Rickettsia siberica
Viral Agents (other than arboviruses)
Hepatitis A Virus, Hepatitis E Virus
Hepatitis B Virus, Hepatitis C Virus (formerly known as nonA nonB Virus), Hepatitis D Virus
Herpesvirus simiae (B-virus)
Human Herpesviruses
Influenza
Lymphocytic Choriomeningitis Virus
Poliovirus
Poxviruses
Rabies Virus
Retroviruses, including Human and Simian Immunodeficiency Viruses (HIV and SIV)
Transmissible Spongiform Encephalopathies (Creutzfeldt-Jakob, kuru, and related agents)
Vesicular Stomatitis Virus
Arboviruses and Arenaviruses - see the following tables:
Table 7.3 Arboviruses and Arenaviruses Assigned to Biosafety Level 2
Table 7.4 Vaccine Strains of BSL 3/4 Viruses Which May be Handled at BSL2
Table 7.5 Arboviruses and Certain Other Viruses Assigned to Biosafety Level 3 (on the basis of
insufficient experience)
Table 7.6 Arboviruses and Certain Other Viruses Assigned to Biosafety Level 3
TABLE 7.3 ARBOVIRUSES AND ARENAVIRUSES ASSIGNED TO BIOSAFETY
LEVEL 2
Acado
Acara
Aguacate
Alfuy
Almpiwar
Amapari
Ananindeua
Anhanga
Anhembi
Anopheles A
Anopheles B
Apeu
Apoi
Aride
Arkonam
Aroa
Aruac
Arumowot
Aura
Avalon
Abras
Abu Hammad
Aabahoyo
Bagaza
Bahig
Bakau
Baku
Bandia
Bangoran
Bangui
Banzi
Barmah Forest
Barur
Batai
Batama
Bauline
Bebaru
Belmont
Benevides
Benfica
Bertioga
Bimiti
Birao
Bluetongue
Boraceia
Botambi
Boteke
Bouboui
Bujaru
Bunyamwera
Bunyip
Burg E Arab
Bushbush
Bussuquara
Buttonwillow
Bwamba
Cacao
Cache Valley
Caimito
California
encephalitis
Calovo
Candiru
Cape Wrath
Capim
Caraparu
Carey Island
Catu
152
Chaco
Chagres
Chandipura
Changuinola
Charleville
Chenuda
Chilibre
Chobar gorge
Clo Mor
Colorado tick fever
Corriparta
Cotia
Cowbone Ridge
Csiro Village
Cuiaba-
D'aguilar
Dakar Bat
Dengue-1
Dengue-2
Dengue-3
Dengue-4
Dera Ghazi Khan
East equine
encephalitis
*
Edge Hill
Entebbe Bat
Ep. Hem. Disease
Erve
Eubenangee
Eyach
Flanders
Fort Morgan
Frijoles
Gamboa
Gan Gan
Gomoka
Gossas
Grand Arbaud
Great Island
Guajara
Guama
Guaratuba
Guaroa
Gumbo Limbo
Hart Park
Hazara
Highlands J
Huacho
Hughes
Icoaraci
Ieri
Ilesha
Ilheus
Ingwavuma
Inkoo
Ippy
Irituia
Isfahan
Itaporanga
Itaqui
Jamestown Canyon
Japanaut
Jerry Slough
Johnston Atoll
Joinjakaka
Juan Diaz
Jugra
Jurona
Jutiapa
Kadam
Kaeng Khoi
Kaikalur
Kaisodi
Kamese
Kammavan pettai
Kannaman galam
Kao Shuan
Karimabad
Karshi
Kasba
Kemerovo
Kern Canyon
Ketapang
Keterah
Keuraliba
Keystone
Kismayo
Klamath
Kokobera
Kolongo
Koongol
Kotonkan
Kowanyama
Kunjin
Kununurra
Kwatta
La Crosse
La Joya
Lagos Bat
Landjia
Langat
Lanjan
Las Maloyas
Latino
Le Dantec
Lebombo
Lednice
Lipovnik
Lokern
Lone Star
Lukuni
M'poko
Madrid
Maguari
Mahogany
Hammock
Main Drain
Malakal
Manawa
Manzanilla
Mapputta
Maprik
Marco
Marituba
Marrakai
Matariya
Matruh
Matucare
Melao
Mermet
Minatitlan
Minnal
Mirim
Mitchell River
Modoc
Moju
Mono Lake
Mont. myotis leuk.
Moriche
Mosqueiro
Mossuril
Mount Elgon Bat
Murutucu
Mykines
Navarro
Nepuyo
Ngaingan
Nique
Nkolbisson
Nola
Ntaya
Nugget
Nyamanini
Nyando
O'nyong-nyong
Okhotskiy
Okola
Olifantsvlei
Oriboca
Ossa
Pacora
Pacui
Pahayokee
Palyam
Parana
Pata
Pathum Thani
Patois
Phnom-Penh Bat
Pichinde
Pixuna
Pongola
Ponteves
Precarious
Point
Pretoria
153
Prospect Hill
Puchong
Punta Salinas
Punta Toro
Qalyub
Quaranfil
Restan
Rio Bravo
Rio Grande
Ross River
Royal Farm
Sabo
Saboya
Saint Floris
Sakhalin
Salehabad
San angelo
Sandfly f. (Naples)
Sandfly f. (Sicilian)
Sandjimba
Sango
Sathuperi
Sawgrass
Sebokele
Seletar
Sembalam
Serra do Navio
Shamonda
Shark River
Shuni
Silverwater
Simbu
Simian hem. fever
Sindbis
Sixgun City
Snowshoe Hare
Sokuluk
Soldado
Sororoca
Stratford
Sunday Canyon
Tacaiuma
Tacaribe
Taggert
Tahyna
Tamiami
Tanga
Tanjong Rabok
Tataguine
Tehran
Tembe
Tembusu
Tensaw
Tete
Tettnang
Thimiri
Thottapalayam
Tibrogargan
Timbo
Timboteua
Tindholmur
Toscana
Toure
Tribec
Triniti
Trivittatus
Trubanaman
Tsuruse
Turlock
Tyuleniy
Uganda S
Umatilla
Umbre
Una
Upolu
Urucuri
Usutu
Uukuniemi
Vellore
Venkatapuram
Vinces
Virgin River
VS-Indiana
VS-New Jersey
Wad Medani
Wallal
Wanowrie
Warrego
Westequine
encephalitis
*
Whataroa
Witwatersrand
Wonga
Wongorr
Wyeomyia
Yaquinea Head
Yata
Yogue
Zaliv Terpeniya
Zegla
Zika
Zingilamo
Zirqa

154
*
A vaccine is available and is recommended for all persons working with this agent.
TABLE 7.4 VACCINE STRAINS OF BSL 3/4 VIRUSES WHICH MAY BE HANDLED
AT BLS2
Virus Vaccine Strain
Chikungunya 131/25
Junin Candid #1
Rift Valley Fever MP-12
Venezuelan equine encephalomyelitis TC-83
Yellow fever 17-D
TABLE 7.5 ARBOVIRUSES AND CERTAIN OTHER VIRUSES ASSIGNED TO
BIOSAFETY LEVEL 3 (ON THE BASIS OF INSUFFICIENT EXPERIENCE)
Adelaide River
Agua Preta
Alenquer
Almeirim
Altamira
Andasibe
Antequera
Araguari
Aransas Bay
Arbia
Arboledas
Babanki
Batken
Belem
Berrimah
Bimbo
Bobaya
Bobia
Bozo
Buenaventura
Cabassue
Cacipacore
Calchaqui
Cananeia
Caninde
Chim
Coastal Plains
Connecticut
Corfou
Dabakala
Douglas
Enseada
Estero Real
Fomede
Forecariah
Fort Sherman
Gabek Forest
Gadgets Gully
Garba
Gordil
Gray Lodge
Gurupi
Iaco
Ibaraki
Ife
Ingangapi
Inini
Issyk-Kul
155
Itaituba
Itimirim
Itupiranga
Jacareacanga
Jamanxi
Jari
Kedougou
Khasan
Kindia
Kyzylagach
Lake Clarendon
Llano Seco
Macaua
Mapuera
Mboke
Meaban
Mojui Dos Compos
Monte Dourado
Munguba
Naranjal
Nariva
Nasoule
Ndelle
New Minto
Ngari
Ngoupe
Nodamura
Northway
Odrenisrou
Omo
Oriximina
Ouango
Oubangui
Oubi
Ourem
Palestina
Para
Paramushir
Paroo River
Perinet
Petevo
Picola
Playas
Pueblo Viejo
Purus
Radi
Razdan
Resistencia
Rochambeau
Salanga
San Juan
Santa Rosa
Santarem
Saraca
Saumarez Reef
Sedlec
Sena Madureira
Sepik
Shokwe
Slovakia
Somone
Spipur
Tai
Tamdy
Telok Forest
Termeil
Thiafora
Tilligerry
Tinaroo
Tlacotalpan
Tonate
Ttinga
Xiburema
Yacaaba
Yaounde
Yoka
Yug Bogkanovac

156
TABLE 7.6 ARBOVIRUSES AND CERTAIN OTHER VIRUSES ASSIGNED TO
BIOSAFETY LEVEL 3
Aino
Akabane
Bhanja
Chikungunya (c,d)
Cocal
Dhori
Dugbe
Everglades (c,d)
Flexal
Germiston (c)
Getah
Hantaan
Israel Turkey mening.
Japanese enc.
Junin (c,d)
Kairi
Kimberley
Koutango
Louping III (a,c)
Mayaro
Middelburg
Mobala
Mopeia (e)
Mucambo (c,d)
Murray Valley enc.
Nairobi sheep disease (a)
Ndumu
Negishi
Oropouche (c)
Orungo
Peaton
Piry
Powassan
Puumala
Rift Valley fever (a,b,c,d)
Sagiyama
Sal Vieja
San Perlita
Semliki Forest
Seoul
Spondweni
St. Louis enc.
Thogoto
Tocio (c)
Turuna
Venezuelan equine
encephalitis (c,d)
Vesicular Stomatitus
(alagoas)
Wesselsbron (a,c)
West Nile
Yellow fever (c,d)
Zinga (b)
a. The importation, possession, or use of this agent is restricted by USDA
regulation or administrative policy.
b. Zinga virus is now recognized as being identical to Rift Valley Fever virus.
c. SALS recommends that work with this agent should be conducted only in
Biosafety Level 3 facilities that provide for HEPA filtration of all exhaust air
prior to discharge from the laboratory.
d. A vaccine is available and is recommended for all persons working with this
agent.
e. This virus is presently being registered in the Catalogue of Arboviruses.

158
8.0 Research Involving Animals
Research involving live animals introduces additional new hazards into the workplace. These hazards are in addition to the
chemical, biological, radiological and physical hazards previously discussed.
8.1 Physical Hazards
Bites
Scratches
Kicks
Crushing or pinning injuries (large animals)
Protocol-associated hazards
8.2 Allergens – Allergic reactions to animals are among the most common conditions that adversely affect the health
of workers involved in the case and use of animals in research. The estimated prevalence of allergic symptoms in the general
population of regularly exposed animal-care workers ranges from 10% to 44%. An estimated 10% of workers eventually
develop occupation-related asthma, in some cases requiring them to find a new vocation. Persons with pre-existing allergies
such as allergic rhinitis (hay fever) are up to 73% more likely to develop allergy to animals. These allergies are usually
manifested as nasal symptoms, itchy eyes, and rashes, but can be more severe in some cases
8.3 Zoonoses
– Zoonoses refer to any infectious disease that can be transmitted from animals (wild or domestic) to
humans, or from humans to animals. They may be bacterial, rickettsial, bacterial, protozoal, fungal, or caused by parasites.
Symptoms and physiological effects may range from non-existent to life-threatening. Knowing the zoonoses associated with
any given animal species, the route of exposure and symptoms is quite beneficial in identifying and controlling worker
exposure to these agents.
Source: Occupational Health and Safety in the Care and Use of Research Animals, National Research Council, 1997.
159
160
APPENDIX A
FACT SHEETS
161
162
EMERGENCY RESPONSE TRAINING FACT SHEET
Based on Title 29 of the Code of Federal Regulations (29 CFR) 1910.120, Hazardous waste operations and emergency response.
____________________________________________
CHEMICAL SPILLS YOU CAN HANDLE YOURSELF
Principal investigators, employees, and students working in research labs should be aware that required safety training for lab workers
includes emergency response training
Emergency training applies to building evacuation procedures during fires and explosions, recognition of system alarms, and
appropriate action in the event of spills of hazardous materials in the lab. Lab workers must receive training to distinguish between the
types of spills they can handle on their own and those spills that are classified as “MAJOR.” Major spills dictate the need for outside
help.
Lab workers are qualified to clean-up spills that are “minor.” A minor spill is defined as a spill that does not pose a significant safety
or health hazard to employees in the immediate vicinity nor does it have the potential to become an emergency within a short time
frame. Lab workers can handle minor spills because they are expected to be familiar with the hazards of the chemicals they routinely
handle during an “average” workday. If the spill exceeds the scope of the lab workers’ experience, training or willingness to respond,
the workers must be able to determine when outside help is necessary.
Emergency assistance is provided by EHS and the University Hazardous Materials Team. Spills requiring the involvement of
individuals outside the lab are those exceeding the exposure one would expect during the normal course of work. Spills in this category
are those which have truly become emergency situations in that lab workers are overwhelmed beyond their level of training. Their
response capability is compromised by the magnitude of the incident. Emergencies such as this involve:
the need to evacuate employees in the area
the need for response from outside the immediate release area
the release poses, or has potential to pose, conditions that are immediately dangerous to life and health
the release poses a serious threat of fire and explosion
the release requires immediate attention due to imminent danger
the release may cause high levels of exposure to toxic substances
there is uncertainty that the worker can handle the severity of the hazard with the personal protective equipment (PPE) and
equipment that has been provided and the exposure limit could be exceeded easily
the situation is unclear or data is lacking regarding important factors.
Depending on the circumstances, what begins as a minor spill could at some point escalate into a major emergency. Responding lab
workers must monitor changing conditions. Again, lab-specific training must cover how to tell the difference!
EHS employees have received in-depth training qualifying them for emergency response beyond the level of minor spills. They are
prepared to answer calls which exceed the training scope of lab workers. Lab workers are encouraged to play it safe and contact EHS
for clean-up assistance when in doubt about the status of a spill. EHS assistance is available 24 hours a day, seven days a week.
EHS: 814-865-6391
____________________________________________
ALL SPILLS THAT REQUIRE OUTSIDE INTERVENTION
A. Emergency Response Procedures. Call 911 to report fires, explosions, medical emergencies, and hazardous material spills.
Dispatch will contact EHS and appropriate emergency response personnel at anytime to respond to hazardous material spills.
An Incident Report form must be completed for each emergency incident involving laboratories.
Following a “MAJOR” incident, EHS responders may determine, based on the circumstances of the spill or release, that clean-up
of the site can be handled by lab workers or other University employees (under the direction of the lab supervisor or EHS

163
In the event that EHS is called to a “minor” spill (i.e., lab workers have been conservative in assessing hazard and assumed worst
case), EHS representatives will participate in or oversee the clean-up to support the lab workers. In both of these cases where
clean-up becomes a lab responsibility, EHS can provide clean-up supplies and equipment, personal protective equipment (to the
level of training of the workers), and safety instructions.
____________________________________________
GENERAL UNIVERSITY EMERGENCY INFORMATION
A. Building Emergency and Evacuation.
In the event of a fire, hazardous material release, or other hazardous situation requiring emergency response, the person who
discovers the emergency will:
evacuate the zone
activate the fire alarm, if needed
call Police Services and report the incident
assist emergency personnel by providing information regarding location of the incident, origin, and persons involved.
The person who discovers the emergency shall not be placed in imminent danger.
C. Incident (Accident) Reporting. All laboratory incidents shall be reported to EHS, including minor spills, fires, or injuries.
Laboratory incidents shall be investigated. The supervisor shall be responsible for implementing corrective action to prevent
repeat incidents.
In the event of worker injury, the immediate supervisor of the injured employee must fill out the First Report of Injury
D. Signs. The following signs and labels are required for all laboratories in University facilities:
A “Laboratory Information” sign shall be posted outside all laboratories, either on the outside of the door or on the wall beside
the door. This sign provides information on specific hazards in the lab and telephone numbers of responsible faculty and staff.
The information shall be updated as necessary.
An “Emergency and Laboratory Safety Phone numbers” sign shall be posted in a prominent location inside the lab, near the
door or telephone. This sign provides emergency numbers in case of an emergency, available from EHS.
A label bearing the University Police emergency number shall be placed on each telephone in the lab, available from EHS.

CHEMICAL SAFETY FACT SHEET
University Safety Policies Storage, Dispensing and Use of Flammable Liquids Policy (SY08)
Hazardous Waste Disposal (SY20)
Based on 29 CFR 1910.1450, Occupational exposure to hazardous chemicals in laboratories.
____________________________________________
EXPOSURE
This section describes remedies for personal exposure to chemicals by inhalation, ingestion, inoculation, or dermal or eye contact.
Additional first aid information for specific chemicals is available from EHS. General procedures are as follows.
POISON CONTROL Phone (800) 942-5969
Inhalation: Get to a source of fresh air. Seek immediate medical assistance.
Ingestion: Seek immediate medical assistance. Never give an unconscious person anything to drink. Do not neutralize acids or bases.
Do not induce vomiting of acid or bases or other solvents unless advised by Poison Control.
Injection: Obtain medical treatment immediately.
Dermal Contact: Obtain immediate medical treatment. Remove the victim from the source of the contamination. Remove
contaminated clothing, cutting it away if necessary. The first aid kit should contain scissors with blunted shear tips for this purpose.
Immediately wash affected areas with water for at least 15 minutes, except in the event of hydrofluoric (HF) acid exposure. For HF
spills, flush for a maximum of 5 minutes. Use the calcium gluconate as soon as possible.
Eye Contact: Obtain immediate medical treatment. Wash eye(s) with water until medical help arrives. Keep the affected eye lower
than the unaffected eye to prevent the spread of contamination. Sterile eyewash cups or irrigator loops are commercially available to
assist in opening the eyelids without prying or traumatizing the injured eye and causing excess pain. These devices can augment
washing of the central portion of the cornea and the superior cul-de-sac where particulate materials may become lodged (thus forming
a solid mass).
____________________________________________
SPILLS
This section describes the procedures for decontamination in the event of a minor chemical spill onto surfaces, materials, instruments,
or equipment. Please address handling of spills of solids and liquids if both are stored in your lab.
Lab workers are responsible for the clean-up of releases that are clearly minor, i.e., do not pose a significant safety or health hazard to
workers in the immediate vicinity or to the worker cleaning the release. Lab workers should not handle spills that have the potential to
become an emergency within a short time. EHS should be contacted for all spills of elemental mercury.
Minor spills are of limited quantity, exposure potential, or toxicity. 911 should be called in the event of an emergency. Lab workers
shall be properly trained to recognize emergency conditions and to notify appropriate responders for situations that are
beyond their own capacity.
When a spill occurs, first cordon off the spill area to prevent inadvertently spreading the contamination over a much larger area.
Pick up small spills of solids with paper towels wetted with water or an appropriate solvent. Solids may be swept up, if harmful
aerosols will not be generated. Place wastes in compatible, sealable containers and dispose of through EHS. Clean instruments or large
areas contaminated with solids with an HEPA filter vacuum cleaner to prevent aerosolization of the contaminant. EHS is available to
provide information and equipment or supervise clean-up.
Wipe up small spills of liquids with paper towels. Use loose absorbent or spill pillows to absorb spills.
Select and wear the appropriate protective gear during clean-up. Basic gear includes lab coat, gloves, and eye protection. Thicker
gloves or double layers may be necessary in some cases. EHS may provide spill equipment if none is present in the lab.

Chemical Safety Fact Sheet, Rev. 1-2000
165
____________________________________________
PROPER WORK AND HANDLING PRACTICES
The following practices are considered standard for use or storage of hazardous chemicals, including carcinogens and reproductive
toxins.
A. Personnel Practices.
1. Eye protection is worn.
2. Gloves are worn for handling hazardous chemicals, including carcinogens, or reproductive toxins.
3. Gloves used are appropriate for the chemicals handled, see glove selection chart.
4. Lab workers wash their hands immediately after removing gloves, after handling chemical agents, and before leaving the lab.
5. Lab coats are worn, fully fastened.
6. Lab coats and gloves are worn only in the lab. They are not taken outside the lab to lunch rooms or offices nor are they worn
outdoors.
7. Following a significant chemical exposure to skin or clothing, lab workers are instructed to use the safety shower
immediately.
8. Eating, drinking, smoking, gum chewing, or applying of cosmetics are prohibited in the work area.
9. Food storage is prohibited in the work area.
10. Food is stored in cabinets or refrigerators designated for such use only.
11. Mechanical pipetting devices are used; mouth pipetting is prohibited.
B. Operational Practices.
1. Hazardous chemicals are used in a chemical fume hood to provide further protection to researchers.
2. All containers of hazardous chemicals are labeled with name of chemical. Abbreviations or formula are not sufficient.
3. Chemical storage is by hazard class. Chemicals are not stored merely by alphabetical order.
4. Chemicals are dated on receipt and opening.
5. Chemicals are removed when the expiration date is exceeded, especially in the case of peroxide-formers.
6. Incompatible materials are physically separated.
7. Flammable materials in amounts exceeding 10 gallons are stored in a flammables storage cabinet.
8. Acids and bases are stored on low shelves or in an acid/base cabinet. Plastic-coated bottles and plastic trays are used to
minimize the effects of leaks.
9. Shock-sensitive, detonable compounds (such as sodium azide, dry picric acid) or extremely poisonous materials (such as
cyanides, osmium tetroxide, cacodylic acid, tetrodotoxin, picrotoxin, ricin) are stored in locked cabinets. DEA-regulated
substances (e.g., pentobarbital, phenobarbital) are also locked in cabinets with keys accessible only to authorized lab workers.
10. Designated work areas are established for handling materials with a high degree of acute toxicity (such as chemicals with
corrosive effects, e.g., nitric, sulfuric and hydrochloric acids, hydrofluoric acid, sodium hydroxide; or chemical asphyxiants
such as carbon monoxide and hydrogen sulfide).
C. Waste Management.
1. A Waste Management Logbook (Appendix B) containing weekly checks, training records, self audits, chemical inventories
and any other waste records and documentation is maintained in all areas where waste is accumulated.
2. All employees and students working with or supervising those working with chemicals or chemical waste must receive
training annually and within 90 days of hire.
3.
A waste accumulation area must be designated near the point of waste generation and posted with the sign available in the
Waste Management Manual.
4. An individual working in the area must be assigned the responsibility for oversight of the accumulation area.
5. Weekly, the area must be checked for the following
Chemicals are not leaking
Chemicals are labeled with red labels provided by EHS. Information on the label includes generator name; chemical
name, amount and concentration; and room and building where generated.
Chemicals are in secondary containment
Chemicals are segregated so that incompatible chemicals are not next to each other.
The total volume of chemicals in the accumulation area does not exceed 55 gal.
6. Wastes are collected in compatible containers which are sealed. Food containers are not appropriate.
7. Sharps (razors, needles, thin pipettes) are collected in puncture-resistant, leakproof containers.

Chemical Safety Fact Sheet, Rev. 1-2000
166
8. Waste pump oil is collected for disposal as hazardous waste.
9. Empty solvent bottles are rinsed 3 times with water and then vented in a chemical fume hood for at least 24 hours. After this
procedure, the bottles may be recycled or thrown in regular trash. All labels must be defaced prior to disposal of the bottle.
Five gal. containers are collected by EHS.
D. Specific Practices for Use with Carcinogens and Reproductive Toxins.
1. Lab surfaces are covered with plastic-backed paper or its equivalent.
2. Procedures involving volatile, powdered, or aerosolized carcinogens are performed in a chemical fume hood that is exhausted
to the outside.
3. Designated work and storage areas are established for carcinogens and reproductive toxins.
4. These areas, including chemical fume hoods and refrigerators, are labeled “Chemical Carcinogen.”
5. Unbreakable outer (secondary) containers are used for transportation of carcinogens.
6. Access procedures are used if work involves moderate or greater amounts of carcinogens or moderate to lengthy procedures.
These procedures may include:
closed doors
restricted access—only authorized personnel permitted
written access procedures posted on the outer door.
7. Dry sweeping and mopping are prohibited if powdered carcinogens or mutagens (e.g., acrylamide and ethidium bromide) are
used.
8. Waste containers for carcinogens are labeled as follows: “Cancer Hazard,” compound name, concentration, and amount.
9. Solid wastes (e.g., pipette tips, gloves, lab paper) are collected in plastic bags, which are sealed and enclosed in a second bag.
The bags are labeled as follows: “Cancer Hazard,” compound name, concentration, and amount.
E. OSHA-Specified Cancer-Causing Agents. Reference in section B found in the Unit Specific Plan Form.
alpha-Naphthylamine
Methyl chloromethyl ether
3,3’-Dichlorobenzidine (and its salts)
bis-Chloromethyl ether
beta-Naphthylamine
Benzidine
4-Aminodiphenyl
Ethyleneimine
beta-Propiolactone
2-Acetylaminofluorene
4-Dimethylaminoazobenzene
N-Nitrosodimethylamine
Vinyl chloride
Inorganic arsenic
Lead
Cadmium
Benzene
1,2-dibromo-3-chloropropane
Acrylonitrile
Ethylene oxide
Formaldehyde
Methylenedianiline
1,3-Butadiene
Methylene chloride
Asbestos
F. Explanation of Medical Surveillance Provisions. If exposure to an OSHA-specified carcinogen is measured to be above the
action level or the short-term exposure limit (STEL), certain specific regulatory requirements come into play, one of which is a
medical surveillance program. Medical surveillance is intended to determine whether employees are experiencing adverse health
effects from exposure to contaminants. It is to be provided without cost to employees and at a reasonable time and place. The
parameters of the medical examination are contaminant-specific and primarily determined by or under the supervision of a
licensed physician. For example, following a worker’s potential exposure to lead, the occupational physician will order biological
monitoring for blood lead level, as required in the OSHA Lead Standard, but the other exam elements are left to the physician’s
Chemical Safety Fact Sheet, Rev. 1-2000
167
discretion. The OSHA Formaldehyde Standard requires medical questionnaires to be completed by workers with possible
formaldehyde exposure. The physician discerns who needs a physical from reviewing the questionnaires.

COMPRESSED GAS CYLINDERS FACT SHEET
Based on 29 CFR 1910.1450, Occupational exposure to hazardous chemicals in laboratories, by reference to Prudent Practices in the Laboratory,
National Research Council.
University Safety Policy SY-25 Compressed Gas Cylinders
____________________________________________
PROPERTIES AND HAZARDS
Handling compressed gases may be more hazardous than handling solid and liquid materials because of the unique properties of gases.
These properties and their associated hazards are:
pressure hazards causing equipment failure and leakage
rapid diffusion, causing dangerous toxic or anesthetic effects, asphyxiation, and rapid formation of explosive concentrations
low boiling-point materials, cryogenic materials, or liquefied gases causing frostbite
the same hazards as those associated with solid or liquid chemicals, including corrosion, irritation, flammability, and high reactivity.
____________________________________________
PROPER WORK AND HANDLING PRACTICES
A. Storage Practices.
1. The regulator is removed and the valve protection cap is in place when cylinders are stored.
2. Cylinders are situated away from heat and ignition sources.
3. Flammable gases (e.g., hydrogen, carbon monoxide) are stored away from other gases, especially oxidizers (e.g., oxygen and
nitrous oxide).
4. Cylinders are situated away from major traffic flow.
5. Cylinders are maintained in an environment at near-room temperatures. They are not subjected to a temperature greater than
125
o
F or lower than -21
o
F.
6. Flames never come into contact with any part of a compressed gas cylinder.
7. A valve protection cap is left on each cylinder until it has been properly secured in the lab and when it is not in use (after having
been secured).
8. Cylinders are secured in accordance with local fire codes. Cylinders must be secured against a wall or bench with cylinder
clamps, chains, or straps, or are placed in a cylinder stand.
B. Transportation.
1. Large cylinders are transported only on a wheeled cylinder cart. Cylinders are not slid or rolled, since even practiced handlers
can lose control of them.
2. Small cylinders are transported in a manner that protects them from potential damage from falling or striking objects.
C. Use of Cylinders.
1. Lab workers wear eye protection when changing regulators or manipulating tubing or equipment potentially under pressure.
2. Cylinders are situated away from heat and ignition sources.
3. Cylinders are situated away from major traffic flow.
4. Cylinders are maintained in an environment at near-room temperatures. They are not subjected to a temperature greater than
125
o
F or lower than -21
o
F.
5. Flames never come into contact with any part of a compressed gas cylinder.
6. Cylinders are used only with a regulator. Cylinders contain pressures greater than most lab equipment can withstand. Cylinder
users are aware that inadvertent closing of a valve or stop cock or plugging of a line could result in a violent failure of the
apparatus.
7. A regulator and gauge shall be installed at the point of use to show the outlet pressure when the source cylinder is outside of the
lab.
8. Cylinder valves are closed when not in use, if feasible. They are never tampered with, forced, lubricated, or modified.
9. Cylinder leaks are attended to immediately. If a leak persists and/or cannot be controlled by simple adjustment, the supplier and
EHS are contacted immediately. The cylinder is removed to a chemical fume hood or location where the leakage can be
exhausted or diluted and left there until the contents can be disposed of according to manufacturer’s directions.
10. When discharging a gas into a liquid, a trap or suitable check valve is used to prevent liquid from backflowing into the cylinder
or regulator.
11. Cylinders are used only with fittings, valves, regulators, and tubing designated by the manufacturer for the gas being used.
12. Connections are not forced or used with homemade adapters.
13. Incompatible gases linked by a direct potential pathway are protected by check valves or other safety devices appropriate for the
gases being used.
14. Ventilation in the use location is adequate to exhaust potential asphyxiant (e.g., carbon dioxide, helium, nitrogen) releases.
Compressed Gas Cylinders Fact Sheet, Rev. 7-97
169
D. Empty Cylinders.
Note: Cylinders are never truly "empty." Empty cylinders shall be handled in the same manner as full and partially full cylinders.
1. Full and empty cylinders are not manifolded together.
2. Empty cylinders are promptly removed from manifolded systems. (Hazardous suckback can occur when an empty cylinder is
mistakenly attached to a pressurized system.)
3. Empty cylinders are labeled "Empty" or "MT."
4. Valves are closed on empty cylinders, leaving a positive pressure. (This prevents the interior from becoming contaminated.)
5. Valve outlets and protective caps received with the cylinder are replaced on empty cylinders.
6. Whenever possible, empty cylinders should be returned to the manufacturer.
7. Where return is not feasible, a chemical pick up request should be completed to have EHS pick up the empty cylinder.
8. Small empty propane or mapp gas cylinders for handheld torches may be disposed of in the regular trash.
E. Specific Procedures for Corrosive Gases.
1. Corrosive gases are stored only for short periods before use, preferably less than three months. Using small cylinders ensures a
reasonable turnover.
2. Corrosive gases are removed from areas containing instruments or other devices sensitive to corrosion.
3. Storage areas for corrosive gases are as dry as possible.
4. A supply of water is available in case of emergency leaks in corrosive gas cylinders. (Most corrosive gases can be absorbed in
water.)
5. Cylinder valve stems on corrosive gases are manipulated frequently to prevent "freezing."
6. Regulators and valves are closed when corrosive gas cylinders are not in use.
7. Regulators and valves are detached from the cylinder except when it is in frequent use (weekly or daily).
8. When corrosive gases are in use, an eyewash is immediately adjacent to the work area.
9. When corrosive gases are in use, a shower is available in close proximity to the work area. The shower must be within 10
seconds travel time of the gas cylinder.
10. Appropriate gloves are worn by lab workers handling corrosive gases.
F. Specific Procedures for Using Acetylene Gas.
1. Acetylene cylinders are stored upright (because they are partially filled with acetone).
2. Acetylene cylinders that have not stood upright are used only after they have been upright for at least 30 minutes.
3.
The outlet line of acetylene cylinders contains a flash arrestor.
4. Pressures are always maintained below the limit indicated by the red warning line on an acetylene pressure gauge.
5. Appropriate tubing is used with acetylene gas. (Copper tubing forms explosive acetylides and shall not be used.)
G. Specific Procedures for Use with Oxygen.
1. When oxygen is used, the cylinder valve is opened momentarily and then closed to blow dirt from the outlet. The valve outlet of
an oxygen cylinder valve is never wiped or touched; this avoids leaving organic residues that might be ignited by exposure to
high oxygen pressure.
2. Oil or grease is never used on the high-pressure side of oxygen and chlorine cylinders or other cylinders containing oxidizing
material. (Otherwise a fire or explosion could result.)
H. Specific Procedures for Use with Toxic, Flammable, and Pyrophoric Gases.
1. Toxic gases are purchased and stored in the smallest sizes possible.
2. During use and storage, highly toxic gases are located in continuously ventilated gas cabinets or mechanical spaces.
3. A continuous gas monitoring system is available for signaling releases of highly toxic gases.
4. Lecture bottles of highly toxic gases are used in a chemical fume hood.
5. Flash arrestors are present on the cylinder lines leading from flammable gases. When flammable gases are used in conjunction
with oxygen, the flammable gas lines are equipped with backflow protection to prevent mixing of oxygen with the fuel.
6. Fires of pyrophoric or highly combustible gases are not considered extinguished until the source of gas is closed off; otherwise,
it can reignite and cause an explosion.
HIGHLY REACTIVE MATERIALS, HIGH-PRESSURE
R
EACTIONS, OR VACUUM SYSTEMS FACT SHEET
Based on 29 CFR 1910.1450, Occupational exposure to hazardous chemicals in laboratories, by reference to Prudent Practices in the Laboratory,
National Research Council.
____________________________________________
GENERAL OPERATIONAL PRACTICES FOR REACTIVE/EXPLOSIVE HAZARDS/SYSTEMS
Note: There may be overlap between these categories. Compounds may be reactive, may cause system over-pressurization, and may be
used with vacuums. All three areas (highly reactive materials, high-pressure systems, vacuum systems) may apply to one reaction.
1. When working with pyrophoric and other highly reactive materials, a flame resistant, fully buttoned lab coat with sleeves fully
extended to the wrists must be worn at all times.
2. Heat guns are not used for heating if flammable vapors are present. Instead, heating tapes, mantles, or water, steam, or oil baths
are used.
3. If an explosion were to occur, provisions have been made to contain the entire reaction mixture.
4. Dry ice solvent baths are not used for reactive gases.
5. Hot liquids are not brought into sudden contact with lower-boiling liquids.
6. Boiling chips are not added once the heated liquid has exceeded its boiling point.
7. The areas where highly reactive chemicals, high-pressure, or vacuum equipment are used are posted with signs to warn
colleagues of potential danger.
8. When a reaction becomes uncontrollable, heat is removed, the addition of reagents is suspended, nearby lab workers are notified,
and the chemical fume hood sash is closed until the temperature has dropped.
9. Emergency equipment is on hand for reactions that could runaway violently.
10. When appropriate, tongs are used to manipulate highly reactive chemicals to prevent exposure of any part of the body to possible
injury (e.g., when immersing sodium metal in solvents, handling heated crucibles, or removing weighing dishes from ovens).
____________________________________________
HIGHLY REACTIVE MATERIALS
A. Definition. Highly reactive materials are those agents that undergo rapid chemical change causing exothermic or other self-
accelerating reactions when subjected to heat, impact, friction, light, catalysts, or other initiation. These agents are materials that will
detonate or deflagrate. Highly reactive materials encompass (but are not limited to):
Air-reactive chemicals (e.g., palladium or platinum on carbon, platinum oxide, Raney nickel)
Metal hydrides (e.g., lithium aluminum hydride, sodium borohydride)
Cryogenic materials/liquefied gas, supercritical fluids (e.g., oxygen, nitrogen, helium)
Highly water-reactive chemicals (e.g., aluminum bromide, metal hydrides, phosphorus pentachloride, tin tetrachloride, titanium
tetrachloride)
Explosive dusts (e.g., magnesium powder, zinc dust, carbon powder, flowers of sulfur)
Explosives, other (e.g., diazomethane, hydrogen peroxide, hydrogen, chlorine, polymerizing acrolein, trinitrotoluene)
Organic peroxides (e.g., acetyl peroxide, benzoyl peroxide)
Organometallic chemicals and active metals (e.g., trimethyl gallium; sodium, magnesium, lithium, potassium)
Oxidizing agents (e.g., halogens, oxyhalogens, peroxyhalogens, permanganates, nitrates, chromates, persulfates, peroxides)
Perchloric acid and perchlorates (e.g., sodium perchlorate)
Peroxide-forming chemicals (e.g., acrylonitrile, dioxane, ether, isopropanol, tetrahydrofuran)
Polymerization reactions (e.g., acrylate monomers)
Polynitro organic chemicals (e.g., picric acid, dinitrophenylhydrazine, methyl nitronitrosoguanidine)
Pyrophoric chemicals (e.g., boranes, white phosphorus, alkyl metals such as n-butyllithium and dibutyl magnesium)
Shock-sensitive and other unstable chemicals (e.g., acetylides, azides, nitro compounds, organic nitrates, perchlorates).
Note: Many of the above classes of materials overlap with other classes (e.g., organometallics may be pyrophoric). The list is
intended merely to provide guidance for determining whether this section applies to the research in your lab. Exact
classification is not necessary.
B. Operational Practices for Specific Classes of Reactives. The categories listed below are not exhaustive and do not necessarily cover
all possible circumstances that must be controlled.
Cryogenic materials
1. Cryogenic materials are not warmed in closed containers.
Highly Reactive Materials, High-Pressure Reactions, or Vacuum Systems Fact Sheet, Rev. 1-2000
171
2. Relief devices have been engineered into the containers or closed systems.
3. Dewars are inspected for ice plug formation.
4. Dry leather or other impervious thermal gloves are worn to prevent burns. Potholders may be used to handle cryogenic
containers.
5. Cryogenic materials are not used in a confined space with inadequate ventilation due to the potential for asphyxiation.
6. Transfers of materials are conducted very slowly to minimize boiling and splashing.
Explosive dusts
1. Suspensions of oxidizeable particles are handled wet.
2. The airborne particulates are not exposed to ignition sources.
3. Adequate ventilation has been provided to control the concentration of airborne dusts.
Organometallics and pyrophoric chemicals
1. Where organometallics are used, Class D fire extinguishers or pails of dry powder extinguishing agent or sand are provided.
2. All pyrophorics are used and stored in an inert atmosphere (e.g., under nitrogen or argon).
3. Regulators are set correctly to prevent glassware from being over pressurized with nitrogen or argon.
4. To avoid spills resulting in fires, breakable glass bottles are stored inside a rubber or plastic bottle carrier.
Organic peroxides and peroxide-forming solvents
1. Organic peroxides and peroxide-forming solvents are protected from and stored away from light.
2. Ceramic, plastic, or wooden spatulas are used with organic peroxides. Metal spatulas are never used.
3. Glass containers with screw caps or glass stoppers are not used with organic peroxides.
4. Friction, grinding, or other forms of impact are not permitted near peroxides.
5. Organic peroxides are diluted with inert solvents such as mineral oil to reduce their sensitivity to heat and shock.
6. Liquid organic peroxides are never allowed to freeze, as phase changes increase the sensitivity of these compounds to shock and
heat.
7. Peroxide-forming solvents are checked for the presence of peroxides prior to heating of the solvent and after each month of
storage. Testing may be conducted with instantaneous peroxide indicator strips.
8. Peroxide-forming solvents are disposed of through EHS within six months after opening.
9. Peroxide-forming compounds are stored in a cool, dark, well-ventilated area.
Ether used as an anesthetic
1. Like other peroxide-formers, ether must be stored in a cool, dry, well-ventilated place, out of direct sunlight. It must be
purchased in small containers, no more than is absolutely necessary. It shall be stored as far back on a shelf as possible to
minimize the potential for falling. It should be easy-to-reach to prevent knocking against the container.
2. Ether is checked for peroxides monthly or discarded six months after opening. Peroxide test strips are available from Lab Safety
Supply and other reputable safety supply distributors (e.g., Fisher, Baxter). In compliance with University safety policy, a chart
of test results accompanies the container of ether. It may be posted on the storage area or kept on a clipboard. A lab safety
designate has been assigned responsibility for regular peroxide testing.
3. Both unused ether supplies (older than 6 months) and ether known to contain peroxides must be disposed of through EHS.
Evaporation of ether in a chemical fume hood is forbidden by law, except for residual amounts in an empty can. Disposal down
the drain is also unlawful.
4. Animal carcasses containing ether are stored in explosion-safe refrigerators or freezers where ether vapors cannot ignite.
Oxidizing agents
1. Oxidizing agents are separated from reducing materials, metals, and ordinary combustibles.
2. Oil baths are not used when oxidizing agents are present.
Perchloric acid and perchlorates
1. Organic materials are digested with nitric acid before the addition of perchloric acid.
2. Perchloric acid is heated (i.e., during acid-based digestion) only in a water-washdown laboratory chemical fume hood of
noncombustible construction.
3. Chemical fume hoods in which perchloric acid is heated are inspected frequently for the accumulation of perchlorates. Deposits
are saturated with water and removed.
4. Perchloric acid is never used near, nor stored on, wooden shelves.
5. Perchloric acid is stored in glass bottles on noncombustible (e.g., ceramic or plastic) trays large enough to contain the entire
contents of the bottle.
6. Perchloric acid and perchlorates are never stored with organic materials.
Highly Reactive Materials, High-Pressure Reactions, or Vacuum Systems Fact Sheet, Rev. 1-2000
172
7. Perchloric acid is never heated with sulfuric acid.
Polynitro organic chemicals and shock-sensitive or unstable compounds
1. The stock of polynitro compounds is stored separately from other lab chemicals.
2. Stock is regularly inspected for degradation or dehydration, as these compounds may become more shock-sensitive with age.
3. Polynitro compounds are disposed of through EHS when no longer needed. They are not placed in storage for future use, as they
may become more hazardous over time.
4. When polynitro and shock-sensitive compounds are moved, they are handled by the container bottom and never by the cap or lid.
5. Picric acid is hydrated or kept in solution to reduce sensitivity. It is never allowed to dry out completely.
6. Solid sodium azide, in quantities above 25 g, is dissolved when it arrives in the lab. Solutions of sodium azide do not pose the
danger of shock-sensitivity associated with the solid form; however, the hydrazoic acid generated when the azide is dissolved is
extremely toxic. Therefore, the solution is always prepared inside a chemical fume hood. If not dissolved, solid azide must be
stored in a locked cabinet.
7. Teflon or other nonmetal spatulas are used with solid sodium azide due to its reactivity with metals.
____________________________________________
HIGH-PRESSURE SYSTEMS
A. Definition. High-pressure reactions are those experiments that are carried out at pressures above one atmosphere. This includes most
hydrogenation reactions since explosive oxygen-hydrogen mixtures can be formed as a result of these reactions.
B. Operational Practices.
1. A label on each pressure vessel indicates the maximum allowable working pressure and temperature.
2. Service lines are not connected to any closed apparatus incapable of withstanding the maximum pressure of the service line (air,
water, etc.).
3. All pressure systems are protected with appropriate pressure-relief devices.
4. The pressure-relief device is installed so that the discharge is directed away from the area where a person could be affected.
5. Pressure-relief devices are inspected periodically. Orifices on both sides of the pressure-relief device are checked for obstruction.
6. The lab workers use pressure gauges with pressure ranges about twice the working pressure of the system.
7. Containers, fittings, and other equipment to be used when working with pressure vessels are able to withstand the stresses
imposed by the given pressures and temperatures.
8. Vessels containing solution are not filled above capacity; preferably, the vessel is only half full.
9. The pressure levels of high-pressure devices are monitored periodically as heating proceeds.
____________________________________________
VACUUM SYSTEMS
A. Definition. Vacuum systems include those activities involving mechanical vacuum pumps, building vacuum systems, water aspirators,
or steam aspirators.
Work with vacuum systems poses a substantial danger of injury to the operator from flying glass shrapnel released during an
implosion. Other hazards may include:
the toxicity of the chemicals in the vacuum system
fire following breakage of a flask containing flammable solvents
toxicity from the mercury in manometers and gauges
over- or under-pressurization arising with thermal conductivity gauges
electric shock with hot cathode ionization systems.
B. Operational Practices.
Vacuum apparatus
1. Vessels used in vacuum operations are protected with suitable relief valves (vacuum breaker).
2. A protective shield is placed around evacuated systems.
3. Lab workers wear safety glasses and face shields when working with evacuated systems or setting up such systems.
4. The vacuum system has been arranged to allow the equipment to be moved without transmitting strain to the neck of the flask;
flasks are supported from below as well as by their necks.
Highly Reactive Materials, High-Pressure Reactions, or Vacuum Systems Fact Sheet, Rev. 1-2000
173
5. The vacuum apparatus is well out of the way of traffic to avoid being struck inadvertently.
6. Belt-driven mechanical pumps have been equipped with protective guards to enclose the moving belts.
Capture of contaminants
1. Each vacuum system used for solvent distillation operations is protected by a suitable trapping device (cold trap, filter, liquid
trap) with a backflow check valve.
2. Water, solvents, and corrosive gases are not allowed to be drawn into the building vacuum (house) system.
3. When mechanical vacuum pumps are used with volatile substances, the input line to the pump is fitted with a cold trap to
minimize the amount of volatile materials entering the pump and dissolving in the oil.
4. If particulates could contaminate a vacuum line (e.g., from an inert atmosphere dry box or glovebox), a HEPA filter will be
installed.
5. If pump oil becomes contaminated, it is drained and changed to prevent the exhaust of chemicals into room air.
6. Used pump oil is disposed of through EHS.
7. Records of use are maintained for general-purpose lab pumps in order to forestall cross-contamination or reactive chemical
incompatibility problems.
8. The exhaust from evacuation of volatile, toxic, or corrosive materials is vented to an air exhaust system such as a chemical fume
hood or local exhaust duct.
Vessels
1. Glass vessels used in conjunction with the vacuum system should be checked with polarized light for cracks, scratches, or
etching each time the vessel is used. At minimum, a visual inspection will be conducted.
2. Dewar flasks are wrapped with tape or enclosed in wooden or metal containers.
3. Reduced pressure is never applied to flat-bottomed flasks unless they have been designed for this purpose.
4. Vacuum desiccators are made of borosilicate/Pyrex glass or plastic.
5. Evacuated desiccators are never carried or moved.
6. Lab workers wait to open desiccators until atmospheric pressure has been restored.
7. If rotary evaporators are used, increases in rotation speed and application of vacuum to the flask are gradual.

BIOLOGICAL SAFETY FACT SHEET
University Safety Policy Use of Biohazardous Materials in Research and Instruction (SY24)
____________________________________________
BIOSAFETY LEVEL 2 CONTAINMENT
A. Principles of Biosafety Containment. “Containment” refers to a reliable plan for managing infectious agents in the lab environment
where they are being handled or maintained. The purpose of containment is to reduce or eliminate exposure of lab workers, other
persons, and the outside environment to potentially hazardous agents.
Biosafety Level 2 (BL2) practices, equipment, and facilities are applicable to clinical, diagnostic, teaching and other facilities in
which work is performed with the broad spectrum of indigenous moderate-risk agents present in the community and associated with
human disease of varying severity. With good microbiological techniques, such agents can be used safely in activities conducted on
the open bench, provided the potential for producing splashes or aerosols is low. Hepatitis B virus, the salmonellae, and Toxoplasma
spp. are representative of microorganisms assigned to this containment level.
B. Applicability of Biosafety Level 2. The BL2 requirements apply to research work:
studying known infectious agents classified as requiring BL2 precautions by the CDC/NIH in Biosafety in Microbiological
and Biomedical Laboratories (BMBL), and
involving the handling of vertebrate animals experimentally or naturally infected with agents classified as requiring BL2
controls by BMBL.
The biological hazard exists in the potential for autoinoculation, ingestion, or mucous membrane exposure by a worker handling
the agents or infected animals.
In addition, BL2 requirements are prescribed for the handling of human blood, blood products, and other potentially
infectious human-derived materials. The following materials are considered potentially infectious:
Human blood, blood components, and blood products
Semen
Vaginal secretions
Cerebrospinal fluid
Synovial fluid
Pleural fluid
Peritoneal fluid
Amniotic fluid
Saliva in dental procedures
Any body fluid visibly contaminated with blood
All body fluids in situations where it is difficult or impossible to differentiate between body fluids
Any unfixed tissue or organs from a human (living or dead)
HIV-containing cell or tissue cultures, organ cultures, and HIV- or HBV-containing culture medium or other solutions
Blood, organs, or other tissues from experimental animals infected with HIV or HBV
Human albumin
Human tissue culture cell lines (even those that are established).
Note: Human albumin and established human cell lines are exempt from the requirements of the Bloodborne Pathogens Standard if
they can be characterized as free of contamination from human hepatitis viruses, human immunodeficiency viruses, and other
recognized bloodborne pathogens. The standard states that the final determination that human or other animal cell lines in
culture are free of bloodborne pathogens must be made by a biological safety professional or other qualified scientist with
background and experience to review such potential contamination and risk. The professional would be expected to comment,
in writing, on the test methods and molecular technology applied to a cell line sample to identify or screen for latent viruses
capable of infecting humans.
Documentation that given cell lines in use in a lab are not classified as “other potentially infectious materials” should be
available in the labs in the Lab Safety Plan. Documentation may be provided by the cell line distributor or vendor (e.g.,
ATCC) at the point of origin but these records do not generally account for potential contamination during shipping. To meet
the requirements of the law, the principal investigator would need to document that the package was protected from
environmental contamination during transport and arrived at the lab undamaged.
175
____________________________________________
SPILLS
A biological spill shall be followed by prompt action to contain and clean-up the spill. When a spill occurs, warn everyone in the area and
call for assistance as needed. Assess the degree of risk involved in the spill based on:
the volume of material spilled
the potential concentration of organisms in the material spilled
the hazard of the organisms involved
the route of infection of the organisms
the diseases caused by the organisms.
Spills of biological agents can contaminate areas and lead to infection of lab workers. Thus, prevention of exposure is the primary goal in
spill containment and clean-up, exactly as in chemical spills. In evaluating the risks of spill response, consider the potential for generation
of aerosols or droplets.
If an accident is expected to generate droplets or aerosols in the laboratory room atmosphere, the room shall be evacuated immediately.
Doors shall be closed and clothing decontaminated. Call EHS to supervise the clean-up. In general, a 30-minute wait is sufficient for the
droplets to settle and aerosols to be reduced by air changes. Longer waiting periods may be imposed depending on the situation and the
ventilation system in this area. Lab workers and/or EHS will exercise judgment as to the need for outside emergency help in evacuation.
If a spill of a biological agent occurs in a public area, evacuation of the area shall be immediate. The principal investigator will be
responsible for designating the extent of evacuation until EHS or emergency personnel arrive. Remember that prevention of exposure to
hazardous aerosols is of primary importance.
Anyone cleaning a spill wears personal protective equipment (for example, lab coat, shoe covers, gloves, safety glasses, and, possibly,
respiratory protection) to prevent exposure to organisms. An air-purifying negative-pressure respirator with HEPA filter cartridges is
generally adequate protection against inhalation of most biological agents. Only personnel trained and cleared through EHS are permitted
to wear respirators.
____________________________________________
PROPER WORK AND HANDLING PRACTICES
A. Standard Practices.
1. Access to the lab is limited or restricted by the supervisor when work with infectious agents is in progress.
2. Work surfaces are decontaminated once a day and after any spill of infectious material.
3. All infectious wastes are chemically decontaminated or autoclaved before disposal.
4. Lab workers wash their hands immediately after removing gloves, after handling agents, and before leaving the lab.
5. All procedures are performed carefully to minimize the creation of aerosols.
6. Eating, drinking, smoking, gum chewing, or applying of cosmetics are prohibited in the work area.
7. Food storage is prohibited in the work area.
8. Food is stored in cabinets or refrigerators designated for such use only.
9. Mechanical pipetting devices are used; mouth pipetting is prohibited.
10. An insect and rodent control program is in effect.
B. Special Practices.
1. Lab doors are kept closed when experiments are in progress.
2. Contaminated materials that are to be transported in public areas (hallways, etc.) are placed in leak-proof, closed, and labeled or
color-coded containers before removal.
3. Access to the lab is restricted. Persons who are at increased risk of acquiring infection or for whom infection may be unusually
hazardous are not permitted. The supervisor has final responsibility regarding entry and work in the lab. Only persons who are
advised of the potential hazard and who meet entry requirements, such as immunization, may enter the lab.
4. If the infectious agents used require special entry provisions, such as immunization, a hazard warning sign is posted on access
doors to the lab. This includes the universal biohazards symbol, infectious agent, principal investigator's name and phone
number, and special entry requirements.
5. Animals unrelated to research are prohibited in the lab.
176
6. Hypodermic needles and syringes are used only for injection and aspiration of fluids. Needle-locking syringes or disposable
units are used.
7. Used needles and syringes are immediately placed in a puncture-resistant container and chemically decontaminated or
autoclaved before discard or reuse.
8. Extreme caution is used when handling needles and syringes to avoid autoinoculation and generation of aerosols during use.
9. Spills and accidents resulting in overt exposures are reported to the supervisor, and EHS.
C. Primary Barriers.
1. Lab workers wear clothing that protects street clothing. This includes at least one of the following options: lab coats, solid-front
gown, smocks, or uniforms.
2. Lab clothing is worn only inside the lab.
3. Lab clothing that is contaminated is either autoclaved or disinfected with 10% bleach solution prior to laundering.
4. Lab workers wear gloves when handling infectious materials.
5. Gloves are carefully removed and changed when visibly contaminated.
6. Persons coming into indirect contact with potentially infectious materials wear gloves if they have lesions or dermatitis on their
hands.
7. Eye protection is worn when splashes, spray, spatter, or droplets of potentially infectious materials may occur.
8. Face shields, or surgical masks and eye protection, are worn when splashes, spray, spatter, or droplets of potentially infectious
materials may occur.
D. Containment Equipment. The following procedure is conducted only in a physical containment device such as a biological safety
cabinet or a chemical fume hood:
Centrifuging, grinding, blending, vigorous shaking or mixing, sonic disruption, opening infectious-material containers for which
internal pressures may differ from ambient pressures, inoculating animals intranasally, and harvesting infected tissue from
animals or eggs.
Exception: Materials may be centrifuged in the open lab if sealed heads or centrifuge safety cups are used and if the centrifuge
tubes are opened only in a physical containment device.
The following procedure is conducted only in a biological safety cabinet:
Use of high concentrations or large volumes of infectious agents.
Lab workers are to be trained in the proper use of biological safety cabinets, with an emphasis on activities that may disrupt the
inward flow of air through the work opening. Staff are aware that the activities that can cause escape of aerosols include:
repeated insertion and withdrawal of worker's arms
opening and closing lab doors or isolation cubicle
improper placement or operation of equipment or materials in the cabinet
brisk walking past the cabinet during use.
____________________________________________
ADDITIONAL PRACTICES SPECIFIC TO THE USE OF HUMAN BLOOD, BLOOD PRODUCTS
OR
OTHER POTENTIALLY INFECTIOUS MATERIALS
Based on 29 CFR 1910.1030, Bloodborne pathogens.
University Safety Policy SY-24 Use of Biohazardous Materials in Research and Instruction
A. Standard Practices. The requirements of the Bloodborne Pathogens Standard are discussed in the University’s Exposure Control
Plan, entitled Bloodborne Pathogens Program (BPP). A copy of the formal plan should be available in your laboratory Safety and
Research Plan. The plan relates the general elements of the program and the expected protocols that shall apply in your lab.
Any lab worker who may be exposed to human blood or other human products shall:
be included in the group’s exposure determination
practice universal precautions
have the opportunity to receive Hepatitis B vaccination
sign a waiver or consent form for vaccination
be offered postexposure evaluation and follow-up (PEEFU)
be trained to understand the hazards of bloodborne pathogens and how to protect against these hazards.
177
In order to comply with the requirement for universal precautions, the PI shall thoroughly evaluate current lab procedures and
supplies for adequacy. Proper application of universal precautions entails:
proper work practice controls for all work involving blood or other potentially infectious materials
initial and annual training, as required by the BPP
provision of engineering controls, as required by the BPP
proper waste disposal with an adequate supply of appropriate sharps containers and biohazard bags present
posting of warning labels and signs
provision of appropriate personal protective clothing and equipment, as required by the BPP.
As further pertains to PPE, the PI shall:
monitor that such supplies are consistently available
ensure its use through periodic checks
oversee its regular cleaning and/or proper disposal
ensure any necessary repair or replacement.
B. Special Practices and Primary Barriers.
1. When working with human blood, blood products, and other potentially infectious materials, lab workers are prohibited from
bending or shearing needles. Needles shall not be replaced in the guards or removed from the syringe.
2. Waste materials from operations involving human blood, blood products, and other potentially infectious materials are
autoclaved or treated with 10% bleach solution before being discarded in regular trash.
3. Spilled material is treated with 10% bleach solution and wiped up with paper towels by lab workers wearing gloves.
4. All paper towels, gloves, and other supplies used in cleaning the spill are disinfected with bleach.
5. Bleached materials are not autoclaved due to corrosion of the steel equipment by potential generation of chlorine gas.
6. Spills on skin are immediately washed with soap and water.
7. Contaminated clothing is immediately autoclaved or treated with 10% bleach solution.
8. Contaminated clothing is not sent for laundering unless it has been autoclaved or treated with 10% bleach solution.
9. All materials disinfected with 10% bleach solution are in contact with the solution for 10 – 30 minutes.
____________________________________________
RECOMBINANT DNA SAFETY PROGRAM
Research involving recombinant DNA shall comply with the National Institute of Health's Guidelines for Research Involving
Recombinant DNA Molecules (NIH Guidelines) as published in the Federal Register. The recombinant DNA guidelines are applicable
to all recombinant DNA research conducted at or sponsored by an institution that receives any support for recombinant DNA research
from NIH. The purpose of the NIH Guidelines is to specify practices for constructing and handling recombinant DNA molecules, and
organisms and viruses containing recombinant DNA molecules.
NIH defines Recombinant DNA molecules as:
molecules that are constructed outside living cells by joining natural or synthetic DNA segments to DNA molecules that can
replicate in a living cell
molecules that result from the replication of those described above.
Synthetic DNA segments which are likely to yield a potentially harmful polynucleotide or polypeptide (e.g., a toxin or a
pharmacologically active agent) are considered as equivalent to their natural DNA counterpart. If the synthetic DNA segment is not
expressed in vivo as a biologically active polynucleotide or polypeptide product, it is exempt from the NIH guidelines. Genomic DNA
of plants and bacteria that have acquired a transposable element, even if the latter was donated from a recombinant vector no longer
present, are not subject to the NIH guidelines unless the transposon itself contains recombinant DNA.
178
APPENDIX B
WASTE MANAGEMENT LOGBOOK
179
WASTE MANAGEMENT LOGBOOK
____________________________________________
GENERAL INSTRUCTIONS
A. Background
Proper handling of reaction byproducts, surplus and waste chemicals, and contaminated materials is an
important part of laboratory safety procedures. Each laboratory worker is responsible for ensuring that
wastes are handled in a manner that minimizes personal exposure and potential for environmental
contamination.
Each laboratory that generates chemical waste must maintain a Waste Management Logbook where
chemical waste is stored. Below is a listing of the required forms that are to be included in the Logbook;
copies of those forms immediately follow this list.
B. The Waste Management Logbook
Contents:
1. Location, Supervisor and Person Responsible for Oversight
2. Acknowledgement of Worker’s Instructions
3. Copy of signed refresher training sheet
4. Lists all people trained to handle hazardous waste
5. Weekly Chemical Waste Review Log
6. Print-out of CHIMS inventory
7. Annual Self Inspection
8. Satellite Accumulation Area Sign

180
Waste Management
Log Book
Webaddress:www.ehs.psu.edu
Designated Accumulation Area:
Building & Room Number
Location in Room
Supervisor:
E-mail
Individualdes i g n a t e d toensure
proceduresarefollowed:
(LabChemicalHygieneOfficer)
E-mail

181
Laboratory Safety
AcknowledgementofWorkerInstructions
I hereby acknowledge having received instructions on the safety procedures for chemical storage and waste
management, including:
log book documentation
proper labeling
proper segregation
non-leaking chemicals
chemical containers kept closed when not in
use
storage limits under 55 gal.
chemical review and inventory
safety
self audit
secondary containment
waste disposal procedure policy (SY 20)
spill/emergency response procedures
use/selection of PPE (Personal Protective
Equipment)
Specific training topics:
Plan to designate specific location of waste accumulation
Ensure documentation is maintained; check labeling, non-leaking chemicals, storage, and containment limits
weekly
How to fill out chemical disposal form and proper procedure for disposal of chemicals
Segregation of chemicals into categories: flammable, bases, and acids in separate storage pans
Chemicals are labeled and closed when not in use
Storage limit (55 gallons) not exceeded
Spill/ emergency response procedures
Use/selection of PPE (Personal Protective Equipment)
Maintain inventory in CHIMS, review chemicals annually: document date reviewed, whether chemical is
retained or disposed of, and explanation
Self-audit established by your research group.
______________________________________________ ___________________________________________
Name (Last, First, Middle Initial) Signature
______________________________________________ ___________________________________________
Date of Training Trainer

182
Comprehensi veListofIndividualsTrainedtoHandleChemicalsand
Waste
(A completed copy of this form should be provided to your Departmental office)
Name:
First Last
Dept. E-Mail Last 4 digits
PSU ID #
Date of
Training

183
ChemicalWasteAccumulat ionArea:WeeklyReviewLog
Date
Checked
No waste in
Accumulation
Area
Labeled
Properly
Segregated
Properly
Not Leaking
Secondary
Containment
in Place
55 Gal
Storage Limit
Not Exceeded
Eyewash tested
Signature
184
Print out and date a copy of
CHIMS Inventory
All chemicals stored must be reviewed annually. Any retained chemicals must be non-
leaking, labeled and confirmed to be in good condition with plans to be used in upcoming
research.
I have reviewed all chemicals in my laboratories and confirm that they are in good
condition and there are plans to use them in the future.
____________________________________________________________________
PI Signature Date

185
(To be completed annualy in January & submitted to your Dept. Office)
Date: Inspector(s):
Building & rm #: Department:
Yes No NA
A. COMPRESSED GASES:
(1) Are cylinders properly secured in an upright position?
(2) Are stored cylinders tightly capped & kept to a minimum?
(3) Are flammable materials stored a minimum of 20 ft from oxygen cylinders?
(4) Are regulators, connections, and tubing in good condition?
(5) Is flammable gas tubing secured and labeled?
(7) If toxic gases are used, are appropriate leak sensors/alarms in place, regularly
checked, and calibrated?
(8) If toxic gases with poor warning qualities are used (i.e. odorless), are redundant
systems and shutoffs in place?
B. ELECTRICAL EQUIPMENT:
Refrigerators and Freezers:
(1) Are only "explosion proof" or "flammable storage" refrigerators/freezers used
to store flammables?
(2) Are refrigerators/freezers which are not "explosion proof" or "Flammable Storage"
clearly labeled "NO FLAMMABLES ALLOWED"?
(3) Are refrigerators labeled for "CHEMICAL USE ONLY" or "FOOD USE
ONLY" and used accordingly?
(4) Is the interior sound and free of chemical spills or contamination?
(5) Are containers stored within stoppered or tightly closed?
General Equipment:
(1) Is electrical apparatus equipped with ground plugs or properly grounded?
(2) Are extension cords in good condition and free of any splices?
(3) Are extension cords for temporary use only, not overloaded, and no
longer than six feet?
(4) Are two-prong appliances not located directly above or within a five-foot
radius of flammables or sinks?
(5) Are electrical panels free from obstruction?
(6) Are appliances properly grounded?
C. EMERGENCY EQUIPMENT:
Fire Extinguishers:
(1) Are extinguishers in designated locations & are these locations labeled?
(2) Are extinguishers accessible and free from obstructions?
(3) Is the current year and date of last inspection indicated on the tag?
Safety Showers and Eyewashes:
(1) Are showers/eyewashes labeled, accessible, and free from obstructions?
(2) Are eyewashes and drench hoses flushed weekly?
(3) Are safety showers flushed annually?
First Aid:
(1) Are first aid kits stored in designated areas?
(2) Is the kit properly stocked according to University Policy SY-21?
PSU LABORATORY SAFETY INSPECTION FORM

186
Yes No NA
D. HAZARDOUS SUBSTANCES:
Chemical Storage:
(1) Has chemical inventory been updated annually?
(2) Are chemicals dated upon receipt?
(3) Are chemical containers labeled, capped, and in good condition?
(4) Is the storage of chemicals on, above, or next to a desk avoided?
(5) Are all corrosive chemicals stored below "eye level"?
(6) Are chemicals segregated by hazard (organics away from oxidizers,
flammables away from acids)?
(7) Is chemical storage kept to a minimum?
Solvent Storage:
(1) Is excess solvent stored in approved safety cans or solvent storage
cabinets and not placed high on shelving?
(2) Are approved safety cans equipped with self-closing lids and are flame
arrestors intact?
(3) Are safety can lids closed?
(4) Are safety cans/wash bottles properly labeled?
Infectious/Chemical Waste:
(1) Are waste containers labeled and chemical compositions identified?
(2) Are waste areas inspected weekly and documentation maintained?
(3) Is waste stored in secondary containment?
(4)Have waste area overseer and Supervisor been designated?
(5) Is total volume of all chemical waste < 55 gallons?
(6) Has the self audit been conducted annually?
(7) Are biohazard containers properly used where needed (i.e. autoclave
bags, sharps containers)?
Laboratory Hoods/Local Exhaust:
(1) Are exhaust hoods working properly? (Confirm date of last inspection).
(2) Do hood sashes open/close properly and is glass intact?
(3) Is hood free of excess chemical storage/equipment?
(4) Are hood sashes down (panels closed) when not accessing?
E. PROTECTIVE EQUIPMENT:
Personal Equipment:
(1) Are safety glasses with side shields worn as required?
(2) Are substantial shoes worn with no sandals or open toes?
(3) Is protective clothing worn while working at benches?
(4) Are gloves selected and worn according to hazard?
(5) Are chemical splash goggles/face shields worn when appropriate?
Other Equipment:
(1) Is proper protective equipment in place (shields,guards, warning sigs,etc?
(2) Is secondary containment used for Hg use and storage?
General Housekeeping:
(1) Are aisles and exits free from obstructions?
(2) Are benches/shelves not overloaded with unused equipment/chemicals?
(3) Are no combustibles stored within three feet of the ceiling?
(4) Is no damaged glassware in use (i.e. broken or chipped)?
(5) Is lab apparatus properly assembled and used in a safe manner?
(6) Are bicycles not stored in lab?
F. SIGNS:
(1) Are special hazard signs in place (i.e. lasers, cryogenic hazards, biohazards)?
(2) Are lab doors labeled and information up-to-date?

187
Yes No NA
G. TRAINING:
(1) Have lab personnel been instructed in potential hazards and lab safety practices?
(2) Has information been provided on the availability of "Right-to-Know" training?
(3) Have all personnel in lab (including PI) received initial chemical/waste training?
(4) Have all personnel in lab (including PI) completed the mandatory refresher training?
H. VACUUM EQUIPMENT:
(1) Are vacuum pump belt guards in place?
(2) Are glass Dewars wrapped or shielded?
(3) Are protective shatterproof shields in place when vacuum equip. is used?
(4) Are glass desiccators under vacuum stored in metal guards or shielded?
I. SECURITY (PSU POLICY SY-24):
(1) Are radioactive, biohazardous material, and hazardous materials secured from
unauthorized removal?
(2) Is lab complying with Erickson/Schultz letter "Safety and Security Procedures
for Hazardous Materials and Food Processing?"
(3) Is lab familiar with PSU Policy SY-24
Use of Biohazardous Materials in Research
and Instruction
and conducting annual inventory of materials regulated
by this policy?
J. STANDARD OPERATING PROCEDURS:
(1) Are standard operating procedures established & available for hazardous operations?
(2) Have you completed your Unit Specific Plan?
_______________________________________________________________________
Name of Principal Investigator (print) Signature of Principal Investigator
_______________________________________________________________________
Name of Department Head (print) Signature of Department Head

188
SATELLITE ACCUMULATION AREA
Please Post
Do you know your responsibilities for proper handling of hazardous
waste?
Please review the following requirements to ensure that you comply with
environmental regulations and safe handling procedures.
TRAINING: Environmental regulations require training of people who generate or
handle hazardous waste. Training must take place within 90 days of date-of-hire; and
annually thereafter.
Training is offered on a regular schedule by Environmental Health and Safety (EHS). Check EHS
Homepage www.ehs.psu.edu for available dates and times.
______________________________________________________________________________________
CONTAINER LABELING AND SECONDARY CONTAINMENT: All hazardous waste containers
must have a red waste tag at the time waste is first placed into the container. The red Chemical Disposal
Label must accurately identify the content of the container. All containers must also be stored in plastic bins
to catch any leakage.
EHS supplies secondary containers and red tags. Call EHS if you need
supplies or additional information.
______________________________________________________________________________________
CONTAINER CLOSURE: Hazardous waste containers must be closed at all times
during storage, except when waste is being added or removed.
Keep containers closed. Regulations do not permit open funnels in waste
Containers when not in use.
______________________________________________________________________________________
STORAGE: For safety and environmental reasons, hazardous waste must be stored in a
designated "Satellite Accumulation Area". These areas must be inspected weekly for
container leakage, labeling, chemical compatibility and to determine that total volume
does not exceed 55 gallons.
If you have full waste containers, please fill out a Chemical pick up request available
on our web page, www.ehs.psu.edu.
189
190
APPENDIX C
UNIT SPECIFIC PLAN

191
UNIT SPECIFIC PLAN
____________________________________________
GENERAL INSTRUCTIONS
A. Background. At PSU, four documents comprise the PSU Laboratory and Research Safety Plan – the General Safety
Plan, the Waste Management Logbook (WML), Rules and Procedures for the Use of Radioactive Material (RPU) and the
Unit Specific Plan. The WML, GSP and the RPU are the companion pieces to the Unit Specific Plan, which is the lab-
specific portion of the Laboratory and Research Safety Plan Together they cover the most salient information needed by
workers to protect themselves from workplace hazards. Should you have difficulty preparing the Unit Specific Plan,
Please refer to EHS for guidance.
Please complete the Unit Specific Plan Form available on the EHS website. The plan will be reviewed by EHS during
routine safety inspections to evaluate whether the types of hazards in your area have been addressed in the plan.
EHS has developed a Web site to inform faculty, staff, and students of the safety and compliance programs and the
policies and procedures with which they should become familiar while at PSU. This Web site is accessible at
www.ehs.psu.edu. All other EHS documents and programs referred to in this Unit Specific Plan are available through the
EHS Web site.
B. Specifics for Completion. The Unit Specific Plan Form consists of the follow sections:
I. Research Overview
II. Safety Infrastructure
III. Chemical Safety
IV. Biological Safety
V. Animal Related Hazards
VI. Radiation Safety
VII. Safety Precautions in Place
VIII. Certification of Agreement
IX. Appendices
The Research Overview and Certification of Agreement sections are required from every principal investigator who
supervises or has responsibility for a lab or group of labs. Lab workers must read the Unit Specific Plan as an
element of initial lab safety training. Section VII. must be signed by everyone working in the lab. New lab workers
who join the group after the Unit Specific Plan has been finalized must read and sign the form as an element of initial
safety training.
The first segment of the Unit Specific Plan should consist of “Fact Sheets” (located in Appendix A) for the various safety
topics of concern in labs. The Fact Sheets summarize standard University guidelines for prudent practice in research
laboratories. Faculty, staff, and students shall familiarize themselves with the guidelines contained on the following
Fact Sheets:
Emergency Response Training Fact Sheet
Chemical Safety Fact Sheet
Compressed Gas Cylinders Fact Sheet
Highly Reactive Materials, High-Pressure Reactions, or Vacuum Systems Fact Sheet
Biological Safety Fact Sheet

192
Please answer all questions and do not leave any blanks. If a question has no connection to the work in your lab(s),
please write “NA” or “not applicable” next to the answer. You are not confined to the boxes for your responses. If you
wish to add additional information, attach separate sheets for elaboration.
The Unit Specific Plan shall be formally reviewed by the PI on an annual basis. Annually you must complete the
Annual Review form. The Unit Specific Plan shall be kept current between Annual Reviews. Whether seen by EHS or
not, the Unit Specific Plan shall reflect new or modified tasks and procedures which affect occupational exposure and
new or revised employee positions with occupational exposure.
The Unit Specific Plan and its component Fact Sheets shall be made available to lab workers and must be readily
available at all times.
Please call EHS with any questions or comments regarding the Unit Specific Plan process. Thank you for your time and
effort in supporting this important safety tool. EHS will be glad to meet with you if you have difficulty with the plan.
C. Information for Contacting EHS.
6 Eisenhower Parking Deck
University Park, PA
814-865-6391
FAX 814-863-7427
www.ehs.psu.edu
