
Citation: Cortés, V.; Patyk, K.;
Simeone, C.; Johnson, V.; Vega, J.;
Savage, K.; Duncan, C. A Review of
Northern Fur Seal (Callorhinus
ursinus) Literature to Direct Future
Health Monitoring Initiatives. Oceans
2022, 3, 303–318. https://doi.org/
10.3390/oceans3030021
Academic Editor: Alexander Werth
Received: 5 May 2022
Accepted: 5 July 2022
Published: 7 July 2022
Publisher’s Note: MDPI stays neutral
with regard to jurisdictional claims in
published maps and institutional affil-
iations.
Copyright: © 2022 by the authors.
Licensee MDPI, Basel, Switzerland.
This article is an open access article
distributed under the terms and
conditions of the Creative Commons
Attribution (CC BY) license (https://
creativecommons.org/licenses/by/
4.0/).
Review
A Review of Northern Fur Seal (Callorhinus ursinus) Literature
to Direct Future Health Monitoring Initiatives
Valerie Cortés
1
, Kelly Patyk
2
, Claire Simeone
3
, Valerie Johnson
4
, Johanna Vega
5
, Kate Savage
6
and Colleen Duncan
1,
*
1
College of Veterinary Medicine and Biomedical Sciences, Colorado State University,
Fort Collins, CO 80526, USA; [email protected]
2
United States Department of Agriculture, Animal Plant and Health Inspection Service,
Veterinary Services, Strategy and Policy, Center for Epidemiology and Animal Health,
Fort Collins, CO 80526, USA; kelly[email protected]
3
Sea Change Health, Sunnyvale, CA 94086, USA; [email protected]g
4
College of Veterinary Medicine, Michigan State University, East Lansing, MI 48824, USA; [email protected]
5
Animal Emergency and Specialty Center, Reno, NV 89511, USA; [email protected]
6
National Marine Fisheries Service (Affiliate), Juneau, AK 99801, USA; [email protected]
* Correspondence: [email protected]
Abstract:
Northern fur seals (Callorhinus ursinus, NFS) are a vulnerable species broadly distributed
throughout the north Pacific. Although commercial hunting stopped in 1984, the population has
continued to decline for unknown reasons. The goal of this scoping review was to synthesize and
review 50 years of literature relevant to the health of NFS to inform the development of health
surveillance recommendations. Search criteria were developed and applied to three databases,
followed by title and abstract screening and full text review. Articles published between 1 January
1972 and 31 December 2021 were included. Articles were categorized by health determinant, and
further as relating to ten subcategories of disease. Data were summarized descriptively. A total of
148 publications met the criteria for inclusion. Infectious disease reports were common, primarily
relating to metazoan parasite presence. The presence of zoonotic pathogens such as Coxiella burnetii
and Brucella spp. is of public health interest, although a failure to link disease research to individual
animal or population health outcomes was consistent across the literature. A shift away from the
single agent focus of disease programs toward more holistic, health-oriented perspectives will require
broader interdisciplinary collaboration. These findings can inform stakeholders and help them to
prioritize and strategize on future NFS health research efforts.
Keywords: Callorhinus ursinus; health; northern fur seal
1. Introduction
The northern fur seal (Callorhinus ursinus, further NFS) is an otariid (eared seal) that
is broadly distributed throughout the north Pacific Ocean. NFSs are the largest of the fur
seals who, similar to other members of the subfamily Arctocephalinae, exhibit significant
sexual dimorphism; adult males can weigh up to 270 kg while adult females are typically
~50 kg [
1
]. They have relatively long lifespans, up to 18 and 27 years for males and females,
respectively, and the generation length is estimated at ~14 years [
2
,
3
]. The species is highly
pelagic, with animals typically only on land during the breeding (and parturition) season.
There are six different breeding populations, i.e., three in the United States and three
in Russia: San Miguel Island, California; Bogoslof Island, eastern Bering Sea; Pribilof
Islands, Alaska; Commander Islands; Kuril Islands; and Robben Island. The largest
breeding population is on the Pribilof Islands, which supports about half the world’s
NFS population [
4
]. After unregulated sealing ended in the 1950s, the population rose
and was estimated at two million, but hunting of female NFS for their pelts from 1956
Oceans 2022, 3, 303–318. https://doi.org/10.3390/oceans3030021 https://www.mdpi.com/journal/oceans

Oceans 2022, 3 304
to 1968 significantly decreased the Pribilof Islands population [
5
]. Despite the cessation
of commercial hunting in 1984, the population has continued to decline for reasons that
are unknown, although a variety of contributory causes (e.g., entanglement in marine
debris, disease and parasites, nutrition, toxins and pollutants, and predation) have all been
proposed [
1
,
2
]. In 1988, the Pribilof Island population was listed as ‘depleted’ under The
Marine Mammal Protection Act [
6
] and the species is currently ‘vulnerable’ according to
the IUCN red list [1].
In general, studying the health of wildlife populations is challenged by the character-
istics of the animals themselves as well as the environments in which they reside, therefore,
making it difficult to access data or samples [
7
]. Such challenges are particularly apparent
in the study of marine mammals where the complexities of investigations have great poten-
tial to introduce bias or limit the external validity of the study. For example, a review of
infectious disease research in polar bears (Ursus maritimus) found that the most detailed
health information was from captive animals housed in physical locations (e.g., zoos) and
environments markedly different than their natural habitat, while information from wild
populations was overwhelmingly disassociated from any clinical, pathological, or popu-
lation health information [
8
]. Similarly, an extensive review of marine mammal disease
literature from North America highlighted substantial publication biases and protracted
lag times between disease events and information sharing [
9
]. How these biases have
influenced NFS research, and how they may be addressed in the future, is less clear.
The definition and study of health in wildlife populations is a topic of increased
attention. Health was once thought of as ‘the absence of disease’ but more modern concepts
of health employ vulnerability, resilience, sustainability, and population stability [
10
]. In an
effort to understand and apply a more dynamic concept of wildlife health, a determinants
of health wildlife model has been proposed. These determinants of health include: needs
for daily living, biologic endowment, physical and social environment, direct mortality
pressures, and human expectations [
11
]. For a highly pelagic species such as the NFS,
meaningfully assessing health, at least in part, by characteristics of their environment
would be helpful given the logistical hurdles of observing or sampling the animals during
all seasons and life stages.
Aggregation of historical and baseline health and disease information is an important
step in the development of any wildlife surveillance program [
7
]. Scoping reviews are a
particularly useful tool to assess the breadth and type of existing research on a topic, to
identify knowledge gaps, to clarify concepts/definitions in the literature, and to investigate
how research is conducted in a certain field [
12
]. The objective of this project was to
synthesize and review literature relevant to the health of the NFS to inform the development
of health surveillance recommendations.
2. Materials and Methods
An overview of search criteria, inclusions and exclusions in accordance with PRISMA [
13
],
is presented in Figure 1. We searched three electronic databases (PubMed, Web of Science,
and Zoological Record) using the search terms “northern fur seal” OR “Callorhinus ursinus”
OR “northern fur seals”. Specific inclusion criteria included publications between 1 January
1972, (in accordance with [
9
]) and 31 December 2021, and printed in English. Records
were limited to articles published in peer-reviewed journals, with ‘grey literature’ such
as government reports, books, and conference proceedings excluded. We read titles and
abstracts and excluded publications that did not focus on NFS health [
11
] or disease [
9
]
and publications which concentrated on tools and techniques (e.g., tagging, modeling,
and methodologies).
Following the exclusion of duplicates and articles that failed to meet the inclusion
criteria, titles and abstracts of all remaining records were reviewed and categorized in-
dependently by three authors (V.C., J.V., and C.D.) according to how they related to NFS
health or disease. Health categories were based on the determinants of wildlife health
model proposed by Wittrock et al., 2019: needs for daily living (e.g., nutrition and habitat
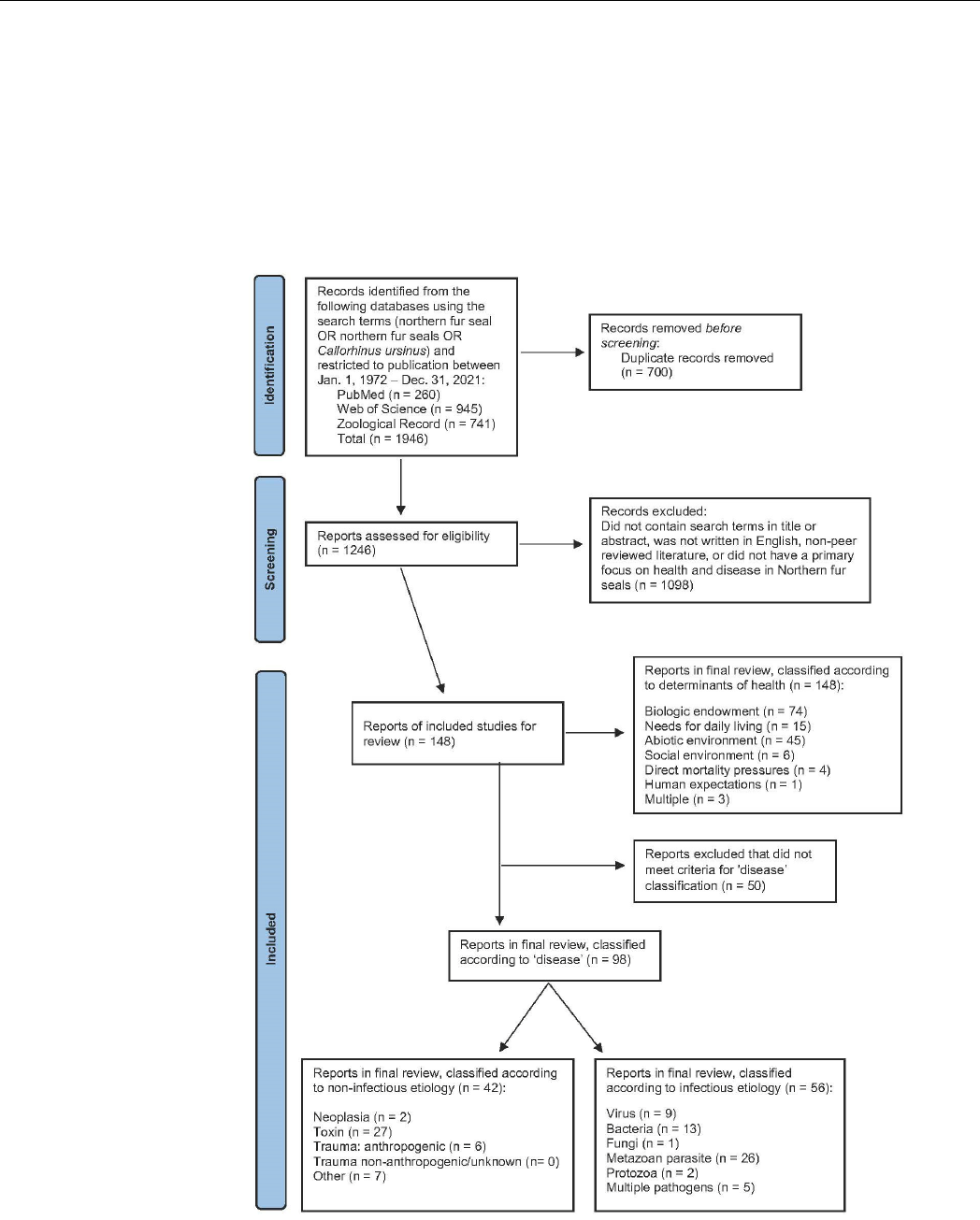
Oceans 2022, 3 305
quality); abiotic environment (e.g., physical surroundings, weather, and water quality);
social environment (e.g., community dynamics, intra- and interspecies interactions); bi-
ologic endowment (e.g., physiological or pathological aspects of wildlife health); direct
mortality pressures (e.g., factors that directly threaten survival); and human expectations
(e.g., service-like entities involved in wildlife or ecosystem management) [
11
]. An article
was classified as relating to disease or not if it covered one of the infectious or non-infectious
disease conditions as described by Simeone et al., 2015 [
9
]. When classifications differed
between authors, consensus was reached through discussion and complete article review
as necessary.
Oceans 2022, 3, FOR PEER REVIEW 3
Figure 1. Schematic representation of the scoping literature process including original searches
through to classification of articles by determinants of health, as well as subcategories of disease.
Following the exclusion of duplicates and articles that failed to meet the inclusion
criteria, titles and abstracts of all remaining records were reviewed and categorized inde-
pendently by three authors (V.C., J.V., and C.D.) according to how they related to NFS
health or disease. Health categories were based on the determinants of wildlife health
model proposed by Wittrock et al., 2019: needs for daily living (e.g., nutrition and habitat
quality); abiotic environment (e.g., physical surroundings, weather, and water quality);
social environment (e.g., community dynamics, intra- and interspecies interactions); bio-
logic endowment (e.g., physiological or pathological aspects of wildlife health); direct
mortality pressures (e.g., factors that directly threaten survival); and human expectations
Figure 1.
Schematic representation of the scoping literature process including original searches
through to classification of articles by determinants of health, as well as subcategories of disease.

Oceans 2022, 3 306
All disease publications were reviewed in full and further divided into 10 subcate-
gories; 5 infectious (viruses, bacteria, fungi, metazoan parasites, and protozoa) and 5 non-
infectious (neoplasia, toxins and contaminants, anthropogenic trauma, non-anthropogenic/
unknown source trauma and ‘other’), similar to classifications by Simeone et al., 2015 [
9
].
Within each of the subcategories, articles were evaluated according to the type of disease
information available (i.e., indirect tests, direct tests, clinical disease/pathology, or other
detectable health impact), the management of the NFS under study (wild or captive) and
the location where the work was done, similar to Fagre et al., 2015 [
8
]. When articles in-
cluded discussion of multiple disease categories that spanned multiple subcategories, they
were assessed within the broader disease review section in addition to acknowledgement
in relevant subcategories.
3. Results
A total of 148 publications met the criteria for inclusion in this study (Figure 1), all of
which could be thematically classified according to the determinants of health. In contrast,
98 publications were classified as ‘disease’.
3.1. Disease Classification
The majority of the disease publications (n = 98) were focused on either a single agent,
or a group of related infectious or non-infectious agents that fit within the specific categories
that follow. A smaller number (n = 5) spanned multiple disparate categories. Most notable
was an extensive case series of post-mortem findings from opportunistically collected NFSs
on St. Paul Island, Alaska, between 1986 and 2006, by Spraker and Lander [
14
]. NFSs were
also included in a large case series describing categories of disease in California stranded
marine mammals from 1984 to 1990 [
15
]. The remaining multi-category publications were
largely infectious disease-oriented and are referenced in relevant subsections below.
3.1.1. Infectious Disease
Viruses
Nine (9.2%) of the disease articles focused on viral diseases. Viral disease was also
described in the necropsy study and referenced in several other of the multi-pathogen
publications [
14
,
16
,
17
]. The majority of these articles, all published prior to 2000, were on
caliciviruses isolated from wild NFS, most commonly in California but also Alaska [
17
–
22
].
Infection was typically devoid of pathology, although vesicular cutaneous lesions have also
been described [
23
]. Proliferative cutaneous lesions associated with pox virus have been
characterized by histopathology and electron microscopy, but were seen very infrequently
relative to the number of animals examined post mortem [14,24].
The remaining reports of viral pathogens were those detected in the reproductive
system. A novel polyomavirus was sequenced from a NFS placenta collected on St. Paul
Island after viral inclusions were seen histologically [
25
]. The single affected placenta
had been opportunistically collected from a rookery and no information was available
regarding fetal or maternal health. There was a single report of Otarine herpes virus 4
detected by polymerase chain reaction (PCR) from vaginal swabs of free ranging NFSs in
Alaska devoid of any associated pathology or description of associated clinical disease [
26
].
Bacteria
Thirteen (13%) of the disease articles focused on a single bacteria and several of the
multi-pathogen articles also included information on bacterial species. The most frequent
bacteria within this group was Coxiella burnetii. In 2010, 75% of the 146 opportunistically
collected NFS placentas from St. Paul Island, Alaska, were PCR positive for C. burnetii and
a subset (3%) of placentas had histologically identified intracytoplasmic bacteria confirmed
as C. burnetii with immunohistochemistry (IHC) [
27
]. A similar molecular prevalence (77%)
was reported in placentas collected from the same rookery the following year [
28
]. Infected
placentas had decreased apoptosis of placental trophoblasts suggesting a functional change

Oceans 2022, 3 307
in the tissue, although no information on the associated NFS pup was available to support
this claim [
29
]. PCR conducted on multiple tissue types from 50 subadult male NFSs
harvested during subsistence hunting were tested for C. burnetii bacterial DNA by real-time
PCR; there were no positive samples [
30
]. Similarly, archived vaginal swabs from adult
female NFSs were all negative [
31
]. A serosurvey using archived samples collected from
animals in the same location revealed high levels of exposure that varied by age class but
appeared to increase between 1994 and 2009/2011 (49–69%) [31].
Brucella spp. have also been identified in the NFS population on St. Paul Island. From
the same collection of placentas tested for C. burnetii above, a single case of necrotizing
placentitis with intracytoplasmic bacteria was seen and attributed to Brucella spp. by IHC
and PCR [
32
]. A total of 119 placentas collected during the 2011 pupping season were
screened by IS711 PCR with 6 (5%) testing positive; and serology using archived samples
suggested a similarly low level of exposure in the population (BMAT 2.5% positive, 30%
borderline) [
32
]. Another serosurvey of 107 archived samples collected in the same area, but
tested by enzyme-linked immunoassay (ELISA), were all negative [
33
]. Fifty subsistence
harvested subadult male NFSs were tested by PCR using eight tissue types, but only a
single spleen sample was positive and no disease was reportedly observed [30].
Pathogen-specific investigations have elucidated information on the presence, and
in some cases pathogenicity, of different bacteria. A variety of Salmonella spp. have been
isolated from the rectums of apparently healthy NFS pups in California and the authors
concluded that the organism could cause opportunistic infections but did not usually cause
disease in healthy animals [
34
]. That said, there was a single case report of a NFS pup
with meningoencephalitis and septicemia where S. enteriditis was cultured from the brain
and spinal cord [
35
]. Serologic screening and post-mortem examinations conducted on
Pribilof Island NFSs in the 1970s revealed a rare leptospiral infection characterized by
interstital nephritis in an adult and multisystem (renal, hepatic and placental) infection
in neonates [
17
,
36
]. Erysipelothrix rhusiopathiae was isolated from the oral cavity of 2/12
otherwise presumed healthy, free-ranging subadult male NFSs on St. Paul Island as part of
a multispecies investigation into the prevalence of the bacteria in the oral cavity of marine
mammals and bite wounds from marine mammals [
37
]. A cross-sectional survey of oral
Pasteurellaceae isolates from captive NFSs and other species concluded that the bacteria are
part of normal marine mammal flora [38].
Multiple tissues have been cultured from subsistance-harvested subadult male NFSs
in Alaska and a variety of mixed bacteria were identified; however, there was no association
with disease and the high apparent prevalence in some normally sterile locations, suggested
a high likelihood of contamination [
39
]. The remaining publications describing bacterial
disease were typically case reports or case series investigations. While routine bacterial
cultures were not conducted as part of the Alaska NFS necropsy program, beta-hemolytic
Escherichia coli was isolated from 11 pups with pneumona [
14
]. Similarly, a captive subadult
male NFS died following a period of anorexia and vomiting and a hemolytic E. coli isolated
from the intestine was determined to be the causative agent [40].
Fungi
Only a single article was identified on fungal disease where Candida albicans was
isolated from asymptomatic NFS in an aquarium setting where phocid seals exhibited
clinical signs associated with infection [41].
Metazoan Parasites
The subcategory with the largest number of infectious disease articles (27%) was
metazoan parasites. This body of research was well summarized in 2021 as an extensive
literature review in addition to describing the intestinal helminth communities from hun-
dreds of additional NFSs [
42
]. While this work explored both spatial and temporal patterns
of infection as well as prevalence and abundance, samples were collected from presumably
healthy animals and, as with the overwhelming majority of the literature on the topic, there

Oceans 2022, 3 308
was little association with pathology or population health impacts. Hookworm (Uncinaria
lucasi) is an exception. The topic of hookworms made up more than half of the metazoan
parasite publications and has been well reviewed by Lyons et al. (2011).The pattern of
disease is variable by region, with hookworms recognized as a major cause of death in
NFS pups in California but uncommon in other sites [
43
]. Gastric lesions associated with
anisakid nematodes were identified in 21% of the stomachs from subadult males harvested
on St. Paul Island, Alaska, 2011–2013; down from 92% as reported from the 1960s [44].
Protozoan Parasites
Two reports of protozoal infection were reviewed. Significant pathology was attributed
to disseminated Toxoplasma gondii (diagnosed by IHC) infection in an adult female NFS that
was stranded in California [
45
]. A brief description of histologically diagnosed sarcocystis
in the muscle of a wild seal found on St. Paul Island was reported in the 1970s, but no
additional information on the animal, or impact on the population, was included [46].
3.1.2. Non-Infectious Disease
Neoplasia
Two case reports focused on neoplastic disease; both reports were on neonates found
dead on the Pribilof Islands, one of which had a renal fibrosarcoma [
47
] and the other
diagnosed with multicentric lymphoma in which viral particles were suspected, but not
confirmed, by electron microscopy [
48
]. A variety of neoplastic processes have been
described by Spraker and Lander including a fetal ganglioneuroblastoma, an adrenal
cortical carcinoma, ovarian dysgerminoma, fibromas, and a squamous papilloma [14].
Toxins and Contaminants
Twenty-eight percent of the disease publications focused on toxins or contaminants.
Overwhelmingly, these publications report contaminant levels in a variety of tissue or
secretory products, but are devoid of any association with individual or population health
or disease information (Table 1). There were two reports on algal toxins, most notably a
case series of stranded, multiple age class NFSs in California that characterized the clinical
and post-mortem disease in NFS with domoic acid poisoning, including central nervous
system signs and pathology of the nervous and cardiac systems consistent with disease in
other species [
49
]. Lower domoic acid and saxitoxin exposures in NFSs relative to other
marine mammals were attributed to differences in foraging behavior [50].
Table 1. A summary of the identified literature on contaminants in NFS.
Contaminant US Japan
Heavy metals (e.g., mercury, cadmium,
arsenic, silver, vanadium)
[51–57] [52,57–60]
Microplastics [61]
Persistent organic pollutants (e.g., PCB,
DDT, PBDEs)
[62–70] [71–74]
Radiocesium [75]
Trauma: Anthropogenic
Of the six articles focused on trauma, all reported traumas attributable to anthro-
pogenic causes. The majority described the frequency and severity of NFS enganglement
in different parts of their geographic range collectively highlighting the hazards of fishing
materials and marine debris [
76
–
79
]. The energetics of entanglement were estimated in
a study on captive animals which highlighted the difficulty that entangled animals can
have both swimming and resting [
80
]. Enganglement as both a cause of death and cause of
observed chronic pathology was also described in the Alaskan necropsy case series [
14
]. A

Oceans 2022, 3 309
single article described the pathology associated with acute head trauma sustained during
NFS harvest activities [81].
Trauma Non-Anthropogenic/Unknown Source
As noted above, all of the disease articles that focused on trauma were classified as
anthropogenic; however, non-anthropogenic or unknown trauma was a very common
finding in the necropsy study in Alaska [
14
]. Trauma in adults was largely the result of
fighting, while, in pups, crushing injuries and bite wounds were both commonly observed.
Other
Seven of the disease papers were classified as ‘other’ by default as they failed to align
with the above criteria, but were broader than the congenital/metabolic category used
by [
9
]. Two of these presumably uncommon disease conditions in captive animals were
written up as case reports. These included an animal with gastric dilatation with volvulus
and a gastric intramural hematoma with hemoperitoneum, both of unknown origin [
82
,
83
].
The remaining articles focused on the ocular or oral cavity. Cross-sectional surveys of
opportunistically collected eyes from both wild and captive NFSs, contributed general
information on gross and histologic changes in NFS eyes, but lacked associated information
on individual or population health impacts [
84
–
86
]. Similarly, two articles described dental
disease and temporomandibular joint pathology in museum collection NFS skulls, but
nothing was reported about the relationship between observed lesions and other causes of
morbidity or mortality [86,87].
An important condition of NFSs that was highlighted in the necropsy case series
from Alaska and California, was emaciation [
14
,
15
]. Emaciation was not only the most
common cause of death in NFS pups on St. Paul Island, but it has reportedly increased over
time [
14
]. A variety of additional conditions such as congenital anomalies, predominantly
musculoskeletal, were described in the longtudinal necropsy study in Alaska [14].
3.2. Health Classification
All of the 148 health and disease publications fit within one of the six determinants
of health categories, although interestingly only 15 (10%) contained the words ‘health’ or
‘healthy’ in the title or abstract. The overwhelming majority (50%) of the articles were
classified as biologic endowment. These were predominantly (89%) the infectious and
non-infectious disease articles described above, with the remaining nine studies focused
on fetal and pup growth and birth weight as related to survival or mortality, as well as
several articles on reproductive indices. Thirty percent of the articles were classified as
abiotic environment. These were largely about exposure to toxins or contaminants in the
environment (n = 27, as included above) or anthropogenic trauma such as entanglements
described above (n = 5). The remaining papers classified as abiotic environment were
not included in any of the disease categories but described important topics such as the
way adverse weather conditions and extreme water temperatures impact seal survival
and dispersal.
All the remaining determinants of health categories were represented, but considerably
less frequently. Needs for daily living (10%) included articles on how foraging behavior,
food web dynamics, prey composition, and selection all affect NFS energetics and overall
success. For example, an article by Short et al. (2021) tied prey availability to NFS pup
survival by showing that commercial pollock fishing in close proximity to the Pribilof
Islands thinned out schools of fish that were normally readily available to lactating female
NFSs, which may have perpetuated low pup survival rates [
88
]. None of these articles was
represented in the disease classification system.
Social environment (4%) also had no overlap with the disease classification system.
These articles were largely investigations into inter- and intraspecific competition for prey,
natal sites, and territoriality. None of these articles were represented in the disease category.
Of particular interest were articles that linked this competition to population trends, such as

Oceans 2022, 3 310
a study by Kuhn et al. (2014) that showed increased densities of NFS may have negative ef-
fects on population growth, due to the increased energy output required to obtain prey [
89
].
Articles classified as direct mortality pressures largely addressed impacts from hunting
and harvesting of NFSs with emphasis on how the sex of harvested seals could impact
population growth. A single study in this category describing pathology associated with
blunt head trauma in harvested seals [
54
], was also classified as traumatic, non-infectious
disease. Only a single article that discussed the relationship between NFS management
and population carrying capacity [
90
] was included in human expectations. Three articles
spanned more than one category and were subsequently classified as ‘multiple’.
4. Discussion
“Absence of evidence is not evidence of absence.”
- Carl Sagan
The presence and absence of information from these 50 years of peer-reviewed NFS
literature can both help to inform research and management programs specific to the
species. Traditionally, such programs are disease focused and based on conditions that
have been observed in the past, and there is a good foundation of NFS disease literature
available to build upon. A particularly noteworthy contribution is the 20-year case series
conducted on St. Paul Island, Alaska, by Spraker and Lander [
14
]. This work involved post-
mortem examination of more than 3000 NFSs, creating a dataset that could be explored for
trends, and could facilitate the collection of biologic samples, generate several hypothesis,
and generally serve as a foundation for several other projects included in this review
(e.g., [
25
,
28
,
29
,
43
,
44
]). Post-mortem examination has been cited as ‘the single most critical
step in diagnosis for general wild animal disease surveillance’ [
91
]. There are several
reasons for this including circumvention of hazards related to capture and handling of live
animals, necropsy as a source of information on variations in ‘normal’ within a species,
identification of several concurrent disease or physiologic changes, and as a sample source
for more targeted disease investigations [
92
–
94
]. The long-term necropsy program on St.
Paul Island is a unique opportunity that could serve as a foundation to which other NFS
health and disease studies should be linked.
If necropsy data is to be used for these purposes however, it is important to understand
both strengths and limitations of the work. The study of pinniped pathology is well known
to be biased by issues of access; overrepresenting animals housed in captive facilities,
species and age groups that strand more commonly, or populations that are easier to
observe and sample [
95
]. Unique access to NFSs highlights this pathology bias. Necropsy
work that has been conducted on St. Paul Island has been made possible by way of a
‘catwalk system’, i.e., raised walkways above some NFS rookeries from which researchers
can safely observe a subset of NFS (those on the rookery) and collect deceased animals using
hooked poles. Limitations to sample collection include size of the deceased animal (light
enough to be picked up) and proximity to the catwalks. Animals included in the Spraker
and Lander paper were overwhelmingly (90%) pups [
14
] which were largely representative
of the number of pups in that location at that time and the lower survival probability of
young animals, however, causes of death and disease are variable by life stage which limits
the external validity of pup necropsy findings. Strategically aligning (e.g., examination,
record keeping, sample collection, testing, and archiving) the necropsy program with other
collection opportunities, such as young adult males harvested for consumption, could
partially help to address this problem. Additionally, as the catwalk system serves as a
sampling transect for the necropsy program, it will be important to ensure it is appropriately
located relative to the rookery. Aerial photos clearly demonstrate changes in the distribution
of NFSs on rookeries as the population declines, resulting in fewer animals adjacent to the
catwalks [
96
,
97
]. Analysis of necropsy findings in conjunction with appropriate population
(‘denominator’) information will help to keep findings in context.
The complete post-mortem examination process is typically overseen by pathologist(s)
and informed by patient history, signalment, clinical disease, and gross necropsy findings.

Oceans 2022, 3 311
The nature and severity of changes seen within an organ system then drive the selection
of additional diagnostic tests to confirm or exclude etiologic agents with the potential to
cause the observed disease. However, because some etiologic agents of concern may not
cause grossly identifiable pathology in any or all animals, routine screening of tissues for
pathogens, toxins, or contaminants may be warranted. Despite numerous publications on
the topic, as highlighted in this review, infectious disease was rarely (3%) implicated as a
cause of pup mortality in the St. Paul Island necropsy study [
14
]. Unfortunately, systematic
screening of tissues for pathogens, toxins, or contaminants, does not appear to have been
conducted. Standardized protocols used to screen for infectious and non-infectious agents
can ensure that the post-mortem examination is sensitive enough to identify any etiologic
agents of concern.
As with biases associated with accessing animals for necropsy, our review highlighted
biases associated with different sample types. The majority of the infectious disease articles
reviewed in this study focused on intestinal parasites, however, with the exception of well
described pathological and epidemiological investigations into hookworm infections [
43
],
this work is largely devoid of associated health or disease information, making it challeng-
ing to tie much of the historical parasitological research into future monitoring programs.
As noted in other reviews of Alaskan wildlife, the overrepresentation of parasites in disease
research is likely, at least in part, to be a function of collection bias as fecal samples are
relatively easy to collect [8].
Similarly, there were many publications on diseases of the NFS placenta (e.g., [
25
,
27
,
29
,
32
]).
Publications on C. burnetii are undoubtably overrepresented in the literature because of
the novelty of this pathogen in the species and geographic location, as well as its potential
risk to humans. However, as with the collection of pups and material for parasitological
investigations, sample availability undoubtably adds additional bias as the opportunity to
systematically collect wild animal placentas is extremely uncommon, and therefore novel.
While several of the pathogens within the NFS placentas have been demonstrated to cause
disease in other species, including humans, use of this sample without any information on
the associated maternal and pup outcomes makes it impossible to link these findings to the
overall health of the population. Awareness of sample biases will be important to consider
in future research efforts.
Failure to link disease research to individual or population outcomes was consistent
across the NFS literature overall. This gap supports arguments for a paradigm shift away
from the siloed, single agent focus of wildlife disease programs to the more holistic, health-
oriented perspective that encompasses the broader social and environmental factors that
are needed in resilient animal populations [
10
,
98
]. Doing this requires strong collaboration
between those with broader (e.g., population, ecosystem) perspectives and experience, and
also recognition that disciplines not classically thought of as ‘health’ domains are, in fact,
most central to this work. Such disciplines may include, but are not limited to, wildlife
(including fisheries) biologists and ecologists, oceanographers, climate scientists, immunol-
ogists, and toxicologists. Through these collaborations, we would be better positioned to
transition the narrative away from looking at sick animals and screening for what is wrong
(disease focus) to looking at healthy populations and identifying characteristics that help
them be well (health focus).
As part of this review we included, and then subclassified, articles based on relevance
to the six determinants of wildlife health proposed by Wittrock et al., 2019 [
11
]. The rank
order of frequency of these in the NFS literature review (biologic endowment, abiotic
environment, and needs for daily living being the top three) were similar to those of
barren ground caribou (Rangifer tarandus groenlandicus) and Pacific salmon (Oncorhyncus
spp.) [
11
]. It should be noted that our search methods (only including the common and
scientific names for NFSs) differed from those of Wittrock et al. and the resulting list of
publications is unlikely to fully represent the scope of research into factors influencing
NFS health. For example, there is undoubtably a vast body of literature on abundance
and distribution of NFS prey species that is relevant to NFS nutrition (‘needs for daily

Oceans 2022, 3 312
living’), but these publications are unlikely to have NFSs as a key word, and therefore,
would not have been identified in our literature search. By restricting full-text review to
articles with the common and/or scientific names for the NFS in the title or abstract we
undoubtably excluded some relevant disease articles as well. That said, it is notable that
our review included peer-reviewed publications spanning all six of the health determinants,
suggesting that this framework may be appropriate for use in a NFS health program in
the future. Interestingly, only 15 (all ‘biologic endowment’ or ‘abiotic environment’) of
the 148 reports in the final review contained the words ‘health’ or ‘healthy’ in the title or
abstract, the majority (n = 12) of which were also classified as disease publications. This
highlights the fact that although the work is relevant to factors influencing the health of
NFSs, numerous researchers and authors may not communicate it that way. Similarly, of
the 148 reports included in our review, 50 publications were determined to be important to
NFS health but were not classified as ‘disease’ articles and half of the health determinant
categories such as (‘needs for daily living’, ‘social environment’, and ‘human expectations‘)
contained no articles that were also captured in the ‘disease’ category. Collectively, this
indicates how much important information could be missed using only a disease centric
approach. Work is needed to engage with individuals and groups working in these more
diverse branches of science that contribute to NFS population ‘health’, covering topics that
include, but are not limited to, ecosystem dynamics, food availability, nutrition, genetic
diversity, and stress.
To address concerns regarding bias and limitations of the historical work, and ways to
better focus on health outcomes, it would be prudent to convene a group of NFS-invested
individuals to develop a strategy for assessing health and disease of NFSs in the future.
As resources (e.g., samples, time, and funding) are finite, it will be necessary to prioritize
indices and conditions upon which to focus. Prioritization models developed and used
successfully in public health livestock and wildlife domains (e.g., [
99
–
103
]) could help
to inform similar efforts for the NFS. The general process involves compiling a list of
conditions for prioritization, selecting appropriate measurement criteria, defining the range
and weighting of levels for each criterion, aggregating scores for each condition, and
ranking conditions by their total score for the final ordering [
104
]. While specific criteria
may vary by species and location, those used in animal health typically include:
1.
General characteristics of the condition in question (e.g., susceptible hosts, reservoirs,
speed of spread, virulence, pathogenicity, and immune response);
2.
Animal health impacts (e.g., morbidity, mortality, reproductive consequences, and
welfare considerations);
3.
Public health impacts (e.g., transmissibility to humans, severity of human disease,
opportunities for human protection, food safety and security, bio/agroterrorism
potential, spread amongst humans, and economic consequences);
4. Regulatory impacts (e.g., local, federal, or international trade consequences);
5. Mitigation (e.g., diagnosis, prevention, and treatment).
Our review has highlighted a body of literature that could aid in the prioritization
process, particularly for diseases of the NFS. For example, several pathogens identified
in this scoping review are zoonotic and some can have significant public health impacts.
Individuals handling marine mammals, including researchers, have unique exposure
opportunities to a variety of organisms they may not normally encounter [105,106]. Some
of the zoonotic pathogens reported in NFSs, such as calicivirus or parapoxvirus, typically
elicit only mild skin lesions, while others such as C. burnetii and Brucella spp., have the
potential to cause more severe systemic disease [
105
,
107
]. Emphasis should be placed on
conditions that are overrepresented in cohorts with increased opportunities for, or evidence
of, exposure such as C. burnetii where the seroprevalence of Alaskan Native residents of the
Pribilof Islands was almost four times the U.S. average [
108
]. Efforts should also be made to
systematically survey for pathogens that may not have not been a topic of significant NFS
research in the past, but are zoonotic, common in sympatric species, and have demonstrated
ability to infect NFSs, such as Toxoplasma or Leptospira [
45
,
95
,
109
]. Engagement with public

Oceans 2022, 3 313
health personnel as part of the prioritization effort is scientifically justified and socially
relevant, but may also be logistically and financially strategic as human and public health
agencies can often access resources (e.g., laboratory expertise and funding sources) not
typically utilized by wildlife professionals.
Similar to the benefits of collaborating with public health professionals, involving those
who study conditions in sympatric species aids in the development of the initial disease
list for use in the prioritization process. This is particularly important for novel animal
health threats where little species-specific information is available; therfore, learning from
others can help inform the development of a surveillance or testing program as necessary.
Spatially clustered health risks (e.g., elevated mercury in Steller sea lions from the western
part of their range [
110
]) could inform targeted data or sample collection. This collaborative
effort may benefit multiple species. For example, infectious diseases such as leptospirosis
or toxoplasmosis occur infrequently in NFSs [
36
,
45
], but can cause devastating disease in
other marine species such as California sea lions and monk seals [
111
,
112
]. As the ecology
of these pathogens is complex, synthesis of information from different species, geographic
regions, and different environmental conditions may elucidate new information.
Finally, this prioritization process needs to be inclusive of all stakeholders and per-
spectives. Wild animals are a public resource. In addition to engaging scientists who
may not already consider themselves a health professional as previously described, the
NFS health prioritization should include members of the public. Involving the public,
even those with little background on the topic, in zoonotic disease prioritization has been
shown to yield meaningful results [
113
]. Northern fur seals are an important subsistence
resource for indigenous people throughout their range, most notably Aleuts [
114
]. The
benefit of incorporating local and traditional knowledge (LTK) is well recognized [
115
–
117
]
and NFS population declines are a sign of changes within the Bering Sea ecosystem that is
well recognized by the local community [
118
]. Frameworks to aid in the systematic and
transparent approach to include LTK in wildlife assessments already exist [
119
]. Inclusivity
of local hunters, fisherman, and indigenous populations with their unique LTK would
make for a more holistic NFS health program.
Working as a team of NFS health and disease experts to prioritize and strategize
on future research efforts would help to address several limitations present in this study.
Our review included only English literature which undoubtably restricted the number
of articles included. NFSs range throughout the North Pacific and research is conducted
in many countries other than the United States. By convening an international team of
NFS researchers and managers, publications identified in this review can be expanded to
include results and perspectives from others that may not be captured here. Similarly, the
smallest of our NFS health determinant categories was human expectations which was also
likely a bias because of our exclusion of ‘grey literature’ in this review. According to [
11
],
this category should include factors such as management policy, education programs,
habitat funding and economics, and traditional knowledge. NFSs are federally managed,
and therefore, inclusion of reports from governing bodies would markedly expand the
available information on this topic. Similarly, the peer-reviewed scientific literature is not a
channel though which LTK is typically shared, re-emphasizing the need to integrate this
information using other established methods [
119
]. Collaboration would also facilitate
the synthesis of ‘negative’ results which could be extremely important information for the
prioritization process. The peer-reviewed literature is biased to focus on novel diseases
and less likely to publish ‘negative’ findings [
9
]. Local people and managing agencies
play a role in approving sample collection and animal handling; their records undoubtably
contain considerably more information than is represented in the peer-reviewed literature.
5. Conclusions
This review of 50 years of scientific literature highlights the fact that NFS health is
more than the presence or absence of innumerable disease-causing agents. Addressing the
complexity of what makes an individual or population healthy requires a system approach

Oceans 2022, 3 314
to look at the many interacting factors (e.g., determinants of health and cumulative effects)
from many different perspectives. Results of this work will be helpful in the next phase of
an inclusive prioritization process that includes strategic planning and establishing mid-
and long-range goals for consistent and targeted assessment and monitoring of NFS health.
Author Contributions:
Conceptualization, C.D., K.P., C.S., K.S. and V.J.; methodology, C.D., K.P., J.V.
and V.C.; formal analysis, J.V. and V.C.; investigation, V.C., K.P., C.S., J.V., V.J., K.S. and C.D.; data
curation, V.C. and J.V.; writing—original draft preparation, C.D., J.V., V.C. and K.P.; writing—review
and editing, V.C., K.P., C.S., J.V., V.J., K.S. and C.D.; visualization, V.C., K.P., J.V. and C.D.; supervision,
C.D.; project administration, C.D.; funding acquisition, C.D., C.S., K.P. and V.J. All authors have read
and agreed to the published version of the manuscript.
Funding:
This research was funded by the NOAA Fisheries AK Region under award NA16NMF4390028
to Colorado State University.
Institutional Review Board Statement:
Not applicable; retrospective literature review that did not
involve live animals.
Informed Consent Statement: Not applicable.
Data Availability Statement: Not applicable.
Acknowledgments:
This work was inspired by thoughtful discussions with our marine mammal
colleagues, particularly Mike Williams, Tom Gelatt, and Rolf Ream. Special thanks also to Maddi
Funk and Ah Young Kim for their assistance preparing our graphical abstract.
Conflicts of Interest:
The sponsors had no role in the design, execution, interpretation, or writing of
the study.
References
1.
Gelatt, T.; Ream, R.; Johnson, D. Callorhinus Ursinus. The IUCN Red List of Threatened Species 2015. Available online: https:
//www.iucnredlist.org/species/3590/45224953 (accessed on 5 May 2022).
2.
Fisheries, N. Northern Fur Seal. Available online: https://www.fisheries.noaa.gov/species/northern-fur-seal#:~{}:text=Male%20
northern%20fur%20seals%20can,territories%20before%20the%20females%20arrive (accessed on 5 May 2022).
3.
Pacifici, M.; Santini, L.; Di Marco, M.; Baisero, D.; Francucci, L.; Grottolo Marasini, G.; Visconti, P.; Rondinini, C. Generation
length for mammals. Nat. Conserv. 2013, 5, 5734. [CrossRef]
4.
Testa, J.W. Fur Seal Investigations, 2015–2016. 2018. Available online: https://doi.org/10.7289/V5/TM-AFSC-375 (accessed on 5
May 2022).
5.
Towell, R.G.; Ream, R.R.; York, A.E. Decline in northern fur seal (Callorhinus ursinus) pup production on the Pribilof Islands. Mar.
Mammal Sci. 2006, 22, 486–491. [CrossRef]
6.
NMFS, Department of Commerce. North Pacific Fur Seal; Pribilof Island Population; Designation as Depleted. Fed. Reg.
1988
, 53,
17888–17899. Available online: https://archives.federalregister.gov/issue_slice/1988/5/18/17881-17909.pdf#page=8 (accessed
on 5 May 2022).
7.
Ryser-Degiorgis, M.-P. Wildlife health investigations: Needs, challenges and recommendations. BMC Vet. Res.
2013
, 9, 223.
[CrossRef] [PubMed]
8.
Fagre, A.C.; Patyk, K.A.; Nol, P.; Atwood, T.C.; Hueffer, K.; Duncan, C.G. A Review of Infectious Agents in Polar Bears (Ursus
maritimus) and Their Long-Term Ecological Relevance. EcoHealth 2015, 12, 528–539. [CrossRef]
9.
Simeone, C.A.; Gulland, F.M.D.; Norris, T.; Rowles, T.K. A Systematic Review of Changes in Marine Mammal Health in North
America, 1972–2012: The Need for a Novel Integrated Approach. PLoS ONE 2015, 10, e0142105. [CrossRef]
10. Stephen, C. Toward a Modernized Definition of Wildlife Health. J. Wildl. Dis. 2014, 50, 427–430. [CrossRef]
11.
Wittrock, J.; Duncan, C.; Stephen, C. A Determinants of Health Conceptural Model for Fish and Wildlife Health. J. Wildl. Dis.
2019, 55, 285–297. [CrossRef]
12.
Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic review or scoping review? Guidance for
authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018, 18, 143. [CrossRef]
13.
Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.;
Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ
2021
, 372, n71.
[CrossRef]
14.
Spraker, T.R.; Lander, M.E. Causes of Mortality in northern Fur Seals (Callorhinus ursinus), St. Paul Island, Pribilof Islands, Alaska,
1986–2006. J. Wildl. Dis. 2010, 46, 450–473. [CrossRef] [PubMed]
15.
Gerber, J.A.; Roletto, J.; Morgan, L.E.; Smith, D.M.; Gage, L.J. Findings in pinnipeds stranded along the central and northern
California coast, 1984–1990. J. Wildl. Dis. 1993, 29, 423–433. [CrossRef] [PubMed]

Oceans 2022, 3 315
16.
Smith, A.W.; Vedros, N.A.; Akers, T.G.; Gilmartin, W.G. Hazards of disease transfer from marine mammals to land mammals:
Review and recent findings. J. Am. Vet. Med. Assoc. 1978, 173, 1131–1133. [PubMed]
17.
Smith, A.W.; Prato, C.M.; Gilmartin, W.G.; Brown, R.J.; Keyes, M.C. A Preliminary Report on Potentially Pathogenic Microbiologi-
cal Agents Recently Isolated from Pinnipeds. J. Wildl. Dis. 1974, 10, 54–59. [CrossRef]
18. Smith, A.W.; Skilling, D.E. Viruses and virus diseases of marine mammals. J. Am. Vet. Med. Assoc. 1979, 175, 918–920.
19.
Barlough, J.E.; Matson, D.O.; Skilling, D.E.; Berke, T.; Berry, E.S.; Brown, R.F.; Smith, A.W. Isolation of Reptilian Calicivirus
Crotalus Type 1 From Feral Pinnipeds. J. Wildl. Dis. 1998, 34, 451–456. [CrossRef]
20.
Sawyer, J.C.; Madin, S.H.; Skilling, D.E. Isolation of San Miguel Sea Lion Virus from Samples of an animal food product produced
from northern fur seal (Callorhinus ursinus) carcasses. Am. J. Vet. Res. 1978, 39, 137–139.
21.
Smith, A.W.; Prato, C.M.; Skilling, D.E. Characterization of two new serotypes of San Miguel sea lion virus. Intervirology
1977
, 8, 30–36.
[CrossRef]
22. Smith, A.W.; Skilling, D.E.; Latham, A.B. Isolation and identification of five new serotypes of calicivirus from marine mammals.
Am. J. Vet. Res. 1981, 42, 693–694.
23.
Prato, C.M.; Akers, T.G.; Smith, A.W. Serological evidence of calicivirus transmission between marine and terrestrial mammals.
Nature 1974, 249, 255–256. [CrossRef]
24.
Hadlow, W.J.; Cheville, N.F.; Jellison, W.L. Occurrence of pox in a northern fur seal on the Pribilof Islands in 1951. J. Wildl. Dis.
1980, 16, 305–312. [CrossRef] [PubMed]
25.
Duncan, C.; Goldstein, T.; Hearne, C.; Gelatt, T.; Spraker, T. Novel polyomaviral infection in the placenta of a northern fur seal
(Callorhinus ursinus) on the Pribilof Islands, Alaska, USA. J. Wildl. Dis. 2013, 49, 163–167. [CrossRef] [PubMed]
26.
Cortés-Hinojosa, G.; Gulland, F.M.D.; DeLong, R.; Gelatt, T.; Archer, L.; Wellehan, J.F.X. A Novel Gammaherpesvirus in Northern
Fur Seals (Callorhinus ursinus) Is Closely Related to the California Sea Lion (Zalophus californianus) Carcinoma-Associated Otarine
Herpesvirus-1. J. Wildl. Dis. 2016, 52, 88–95. [CrossRef] [PubMed]
27.
Duncan, C.; Kersh, G.; Spraker, T.; Patyk, K.; Fitzpatrick, K.; Massung, R.; Gelatt, T. Coxiella burnetii in Northern Fur Seal
(Callorhinus ursinus) Placentas from St. Paul Island, Alaska. Vector-Borne Zoonotic Dis. 2012, 12, 192–195. [CrossRef] [PubMed]
28.
Duncan, C.; Savage, K.; Williams, M.; Dickerson, B.; Kondas, A.V.; Fitzpatrick, K.A.; Guerrero, J.L.; Spraker, T.; Kersh, G.J. Multiple
strains of Coxiella burnetii are present in the environment of St. Paul Island, Alaska. Transbound. Emerg. Dis.
2013
, 60, 345–350.
[CrossRef] [PubMed]
29.
Myers, E.; Ehrhart, E.J.; Charles, B.; Spraker, T.; Gelatt, T.; Duncan, C. Apoptosis in normal and Coxiella burnetii-infected placentas
from Alaskan northern fur seals (Callorhinus ursinus). Vet. Pathol 2013, 50, 622–625. [CrossRef] [PubMed]
30.
Duncan, C.; Dickerson, B.; Pabilonia, K.; Miller, A.; Gelatt, T. Prevalence of Coxiella burnetii and Brucella spp. in tissues from
subsistence harvested northern fur seals (Callorhinus ursinus) of St. Paul Island, Alaska. Acta Vet. Scand.
2014
, 56, 67. [CrossRef]
[PubMed]
31.
Minor, C.; Kersh, G.J.; Gelatt, T.; Kondas, A.V.; Pabilonia, K.L.; Weller, C.B.; Dickerson, B.R.; Duncan, C.G. Coxiella burnetii in
northern fur seals and Steller sea lions of Alaska. J. Wildl. Dis. 2013, 49, 441–446. [CrossRef]
32.
Duncan, C.G.; Tiller, R.; Mathis, D.; Stoddard, R.; Kersh, G.J.; Dickerson, B.; Gelatt, T. Brucella placentitis and seroprevalence in
northern fur seals (Callorhinus ursinus) of the Pribilof Islands, Alaska. J. Vet. Diagn. Investig. 2014, 26, 507–512. [CrossRef]
33.
Nymo, I.H.; Rødven, R.; Beckmen, K.; Larsen, A.K.; Tryland, M.; Quakenbush, L.; Godfroid, J. Brucella Antibodies in Alaskan True
Seals and Eared Seals—Two Different Stories. Front. Vet. Sci. 2018, 5, 8. [CrossRef]
34.
Gilmartin, W.G.; Vainik, P.M.; Neill, V.M. Salmonellae in feral pinnipeds off the Southern California coast. J. Wildl. Dis.
1979
, 15,
511–514. [CrossRef] [PubMed]
35.
Stroud, R.K.; Roelke, M.E. Salmonella meningoencephalomyelitis in a northern fur seal (Callorhinus ursinus). J. Wildl. Dis.
1980
,
16, 15–18. [CrossRef] [PubMed]
36.
Smith, A.W.; Brown, R.J.; Skilling, D.E.; Bray, H.L.; Keyes, M.C. Naturally-occurring leptospirosis in northern fur seals (Callorhinus
ursinus). J. Wildl. Dis. 1977, 13, 144–148. [CrossRef] [PubMed]
37.
Suer, L.; Vedros, N.A. Erysipelothrix rhusiopathiae. I. Isolation and characterization from pinnipeds and bite/ abrasion wounds
in humans. Dis. Aquat. Org. 1988, 5, 1–5. [CrossRef]
38.
Hansen, M.J.; Bertelsen, M.F.; Christensen, H.; Bisgaard, M.; Bojesen, A.M. Occurrence of Pasteurellaceae bacteria in the oral
cavity of selected marine mammal species. J. Zoo Wildl. Med. 2012, 43, 828–835. [CrossRef]
39.
Vedros, N.A.; Quinlivan, J.; Cranford, R. Bacterial and fungal flora of wild northern fur seals (Callorhinus ursinus). J. Wildl. Dis.
1982, 18, 447–456. [CrossRef]
40.
Vanpelt, R.W.; Ohata, C.A. Hemolytic Escherichia coli as Cause of Acute Enterotoxemia in a Captive Northern Fur Seal. Vet. Med.
Small Anim. Clin. 1974, 69, 1251–1254.
41. Dunn, J.L.; Buck, J.D.; Spotte, S. Candidiasis in captive pinnipeds. J. Am. Vet. Med. Assoc. 1984, 185, 1328–1330.
42.
Kuzmina, T.A.; Kuzmin, Y.; Dzeverin, I.; Lisitsyna, O.I.; Spraker, T.R.; Korol, E.M.; Kuchta, R. Review of metazoan parasites of the
northern fur seal (Callorhinus ursinus) and the analysis of the gastrointestinal helminth community of the population on St. Paul
Island, Alaska. Parasitol. Res. 2021, 120, 117–132. [CrossRef]
43.
Lyons, E.T.; Spraker, T.R.; De Long, R.L.; Ionita, M.; Melin, S.R.; Nadler, S.A.; Tolliver, S.C. Review of research on hookworms
(Uncinaria lucasi Stiles, 1901) in northern fur seals (Callorhinus ursinus Linnaeus, 1758). Parasitol. Res.
2011
, 109, 257–265.
[CrossRef]

Oceans 2022, 3 316
44.
Kuzmina, T.A.; Lyons, E.T.; Spraker, T.R. Anisakids (Nematoda: Anisakidae) from stomachs of northern fur seals (Callorhinus
ursinus) on St. Paul Island, Alaska: Parasitological and pathological analysis. Parasitol. Res.
2014
, 113, 4463–4470. [CrossRef]
[PubMed]
45.
Holshuh, H.J.; Sherrod, A.E.; Taylor, C.R.; Andrews, B.F.; Howard, E.B. Toxoplasmosis in a feral northern fur seal. J. Am. Vet. Med.
Assoc. 1985, 187, 1229–1230. [PubMed]
46. Brown, R.J.; Smith, A.W.; Keyes, M.C. Sarcocystis in the northern fur seal. J. Wildl. Dis. 1974, 10, 53. [CrossRef] [PubMed]
47. Brown, R.J.; Smith, A.W.; Keyes, M.C. Renal fibrosarcoma in the northern fur seal. J. Wildl. Dis. 1975, 11, 23–25. [CrossRef]
48.
Stedham, M.A.; Casey, H.W.; Keyes, M.C. Lymphosarcoma in an infant northern fur seal (Callorhinus ursinus). J. Wildl. Dis.
1977
,
13, 176–179. [CrossRef]
49.
Lefebvre, K.A.; Robertson, A.; Frame, E.R.; Colegrove, K.M.; Nance, S.; Baugh, K.A.; Wiedenhoft, H.; Gulland, F.M.D. Clinical
signs and histopathology associated with domoic acid poisoning in northern fur seals (Callorhinus ursinus) and comparison of
toxin detection methods. Harmful Algae 2010, 9, 374–383. [CrossRef]
50.
Lefebvre, K.A.; Quakenbush, L.; Frame, E.; Huntington, K.B.; Sheffield, G.; Stimmelmayr, R.; Bryan, A.; Kendrick, P.; Ziel,
H.; Goldstein, T.; et al. Prevalence of algal toxins in Alaskan marine mammals foraging in a changing arctic and subarctic
environment. Harmful Algae 2016, 55, 13–24. [CrossRef]
51. Beckmen, K.B.; Duffy, L.K.; Zhang, X.; Pitcher, K.W. Mercury concentrations in the fur of Steller sea lions and northern fur seals
from Alaska. Mar. Pollut. Bull. 2002, 44, 1130–1135. [CrossRef]
52.
Noda, K.; Ichihashi, H.; Loughlin, T.R.; Baba, N.; Kiyota, M.; Tatsukawa, R. Distribution of heavy metals in muscle, liver and
kidney of northern fur seal (Callorhinus ursinus) caught off Sanriku, Japan and from the Pribilof Islands, Alaska. Environ. Pollut.
1995, 90, 51–59. [CrossRef]
53.
Goldblatt, C.J.; Anthony, R.G. Heavy Metals in Northern Fur Seals (Callorhinus ursinus) from the Pribilof Islands, Alaska. J.
Environ. Qual. 1983, 12, 478–482. [CrossRef]
54.
Kim, K.C.; Chu, R.C.; Barron, G.P. Mercury in tissues and lice of Northern fur seals. Bull. Environ. Contam. Toxicol.
1974
, 11,
281–284. [CrossRef] [PubMed]
55.
Anas, R.E. Heavy metals in the northern fur seal, Callorhinus ursinus, and harbor seal, Phoca vitulina richardi. Fish. Bull.
1974
, 72,
133–137.
56.
Zeisler, R.; Demiralp, R.; Koster, B.J.; Becker, P.R.; Burow, M.; Ostapczuk, P.; Wise, S.A. Determination of inorganic constituents in
marine mammal tissues. Sci. Total Environ. 1993, 139–140, 365–386. [CrossRef]
57.
Saeki, K.; Nakajima, M.; Noda, K.; Loughlin, T.R.; Baba, N.; Kiyota, M.; Tatsukawa, R.; Calkins, D.G. Vanadium Accumulation in
Pinnipeds. Arch. Environ. Contam. Toxicol. 1999, 36, 81–86. [CrossRef]
58.
Arai, T.; Ikemoto, T.; Hokura, A.; Terada, Y.; Kunito, T.; Tanabe, S.; Nakai, I. Chemical Forms of Mercury and Cadmium
Accumulated in Marine Mammals and Seabirds as Determined by XAFS Analysis. Environ. Sci. Technol.
2004
, 38, 6468–6474.
[CrossRef]
59.
Fujihara, J.; Kunito, T.; Kubota, R.; Tanabe, S. Arsenic accumulation in livers of pinnipeds, seabirds and sea turtles: Subcellular
distribution and interaction between arsenobetaine and glycine betaine. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol.
2003
,
136, 287–296. [CrossRef]
60. Saeki, K.; Nakajima, M.; Loughlin, T.R.; Calkins, D.C.; Baba, N.; Kiyota, M.; Tatsukawa, R. Accumulation of silver in the liver of
three species of pinnipeds. Environ. Pollut. 2001, 112, 19–25. [CrossRef]
61.
Donohue, M.J.; Masura, J.; Gelatt, T.; Ream, R.; Baker, J.D.; Faulhaber, K.; Lerner, D.T. Evaluating exposure of northern fur seals,
Callorhinus ursinus, to microplastic pollution through fecal analysis. Mar. Pollut. Bull. 2019, 138, 213–221. [CrossRef]
62.
Wang, D.; Shelver, W.L.; Atkinson, S.; Mellish, J.A.; Li, Q.X. Tissue distribution of polychlorinated biphenyls and organochlorine
pesticides and potential toxicity to Alaskan northern fur seals assessed using PCBs congener specific mode of action schemes.
Arch. Environ. Contam. Toxicol. 2010, 58, 478–488. [CrossRef]
63. Mössner, S.; Barudio, I.; Spraker, T.S.; Antonelis, G.; Early, G.; Geraci, J.R.; Becker, P.R.; Ballschmiter, K. Determination of HCHs,
PCBs, and DDTs in brain tissues of marine mammals off different age. Fresenius J. Anal. Chem. 1994, 349, 708–716. [CrossRef]
64.
Norstrom, R.J.; Muir, D.C. Chlorinated hydrocarbon contaminants in arctic marine mammals. Sci. Total Environ.
1994
, 154,
107–128. [CrossRef]
65.
Schantz, M.M.; Koster, B.J.; Wise, S.A.; Becker, P.R. Determination of PCBs and chlorinated hydrocarbons in marine mammal
tissues. Sci. Total Environ. 1993, 139–140, 323–345. [CrossRef]
66.
Beckmen, K.B.; Blake, J.E.; Ylitalo, G.M.; Stott, J.L.; O’Hara, T.M. Organochlorine contaminant exposure and associations with
hematological and humoral immune functional assays with dam age as a factor in free-ranging northern fur seal pups (Callorhinus
ursinus). Mar. Pollut. Bull. 2003, 46, 594–606. [CrossRef]
67.
Loughlin, T.R.; Castellini, M.A.; Ylitalo, G. Spatial aspects of organochlorine contamination in northern fur seal tissues. Mar.
Pollut. Bull. 2002, 44, 1024–1034. [CrossRef]
68. Beckmen, K.B.; Ylitalo, G.M.; Towell, R.G.; Krahn, M.M.; O’Hara, T.M.; Blake, J.E. Factors affecting organochlorine contaminant
concentrations in milk and blood of northern fur seal (Callorhinus ursinus) dams and pups from St. George Island, Alaska. Sci.
Total Environ. 1999, 231, 183–200. [CrossRef]

Oceans 2022, 3 317
69.
Mössner, S.; Spraker, T.R.; Becker, P.R.; Ballschmiter, K. Ratios of enantiomers of alpha-HCH and determination of alpha-, beta-,
and gamma-HCH isomers in brain and other tissues of neonatal Northern fur seals (Callorhinus ursinus). Chemosphere
1992
, 24,
1171–1180. [CrossRef]
70.
Reiner, J.L.; Becker, P.R.; Gribble, M.O.; Lynch, J.M.; Moors, A.J.; Ness, J.; Peterson, D.; Pugh, R.S.; Ragland, T.; Rimmer, C.; et al.
Organohalogen Contaminants and Vitamins in Northern Fur Seals (Callorhinus ursinus) Collected During Subsistence Hunts in
Alaska. Arch. Environ. Contam. Toxicol. 2016, 70, 96–105. [CrossRef]
71.
Nomiyama, K.; Kanbara, C.; Ochiai, M.; Eguchi, A.; Mizukawa, H.; Isobe, T.; Matsuishi, T.; Yamada, T.K.; Tanabe, S. Halogenated
phenolic contaminants in the blood of marine mammals from Japanese coastal waters. Mar. Environ. Res.
2014
, 93, 15–22.
[CrossRef]
72.
Tanabe, S.; Sung, J.-K.; Choi, D.-Y.; Baba, N.; Kiyota, M.; Yoshida, K.; Tatsukawa, R. Persistent organochlorine residues in northern
fur seal from the Pacific coast of Japan since 1971. Environ. Pollut. 1994, 85, 305–314. [CrossRef]
73.
Kajiwara, N.; Ueno, D.; Takahashi, A.; Baba, N.; Tanabe, S. Polybrominated diphenyl ethers and organochlorines in archived
northern fur seal samples from the Pacific coast of Japan, 1972–1998. Environ. Sci. Technol. 2004, 38, 3804–3809. [CrossRef]
74.
Iwata, H.; Tanabe, S.; Iida, T.; Baba, N.; Ludwig, J.P.; Tatsukawa, R. Enantioselective Accumulation of
α
-Hexachlorocyclohexane
in Northern Fur Seals and Double-Crested Cormorants: Effects of Biological and Ecological Factors in the Higher Trophic Levels.
Environ. Sci. Technol. 1998, 32, 2244–2249. [CrossRef]
75.
Ruedig, E.; Duncan, C.; Dickerson, B.; Williams, M.; Gelatt, T.; Bell, J.; Johnson, T.E. Fukushima derived radiocesium in
subsistence-consumed northern fur seal and wild celery. J. Environ. Radioact. 2016, 152, 1–7. [CrossRef] [PubMed]
76.
Kuzin, A.E.; Trukhin, A.M. Entanglement of northern fur seals (Callorhinus ursinus) in marine debris on Tyuleniy Island (Sea of
Okhotsk) in 1998–2013. Mar. Pollut. Bull. 2019, 143, 187–192. [CrossRef] [PubMed]
77.
Hanni, K.D.; Pyle, P. Entanglement of Pinnipeds in Synthetic Materials at South-east Farallon Island, California, 1976–1998. Mar.
Pollut. Bull. 2000, 40, 1076–1081. [CrossRef]
78.
Kiyota, M.; Baba, N. Entanglement in marine debris among adult female northern fur seals at St. Paul Island, Alaska in 1991–1999.
Bull.-Natl. Res. Inst. Far Seas Fish. 2001, 38, 13–20.
79.
Stewart, B.S.; Yochem, P.K. Entanglement of pinnipeds in synthetic debris and fishing net and line fragments at San Nicolas and
San Miguel Islands, California, 1978–1986. Mar. Pollut. Bull. 1987, 18, 336–339. [CrossRef]
80.
Feldkamp, D.M.; Costa, D.P.; Dekrey, G.K. Energetic and Behavioral Effects of Net Entanglement on Juvenile Northern Fur Seals,
Callorhinus ursinus. Fish. Bull. 1989, 87, 85–94.
81.
Jortner, B.S. Neuropathologic Observations of Head Trauma in the Northern Fur Seal. J. Wildl. Dis.
1974
, 10, 121–129. [CrossRef]
82.
Frasca, S., Jr.; Van Kruiningen, H.J.; Dunn, J.L.; St Aubin, D.J. Gastric intramural hematoma and hemoperitoneum in a captive
northern fur seal. J. Wildl. Dis. 2000, 36, 565–569. [CrossRef]
83.
Frasca, S., Jr.; Dunn, J.L.; Van Kruiningen, H.J. Acute gastric dilatation with volvulus in a northern fur seal (Callorhinus ursinus). J.
Wildl. Dis. 1996, 32, 548–551. [CrossRef]
84.
Stoskopf, M.K.; Zimmerman, S.; Hirst, L.W.; Green, R. Ocular anterior segment disease in northern fur seals. J. Am. Vet. Med.
Assoc. 1985, 187, 1141–1144. [PubMed]
85.
Miller, S.; Colitz, C.M.H.; St. Leger, J.; Dubielzig, R. A retrospective survey of the ocular histopathology of the pinniped eye with
emphasis on corneal disease. Vet. Ophthalmol. 2013, 16, 119–129. [CrossRef] [PubMed]
86.
Colitz, C.M.H.; Renner, M.S.; Manire, C.A.; Doescher, B.; Schmitt, T.L.; Osborn, S.D.; Croft, L.; Olds, J.; Gehring, E.; Mergl, J.; et al.
Characterization of progressive keratitis in Otariids. Vet. Ophthalmol. 2010, 13, 47–53. [CrossRef] [PubMed]
87.
Aalderink, M.T.; Nguyen, H.P.; Kass, P.H.; Arzi, B.; Verstraete, F.J. Dental and Temporomandibular Joint Pathology of the Northern
Fur Seal (Callorhinus ursinus). J. Comp. Pathol. 2015, 152, 325–334. [CrossRef] [PubMed]
88.
Short, J.W.; Geiger, H.J.; Fritz, L.W.; Warrenchuk, J.J. First-Year Survival of Northern Fur Seals (Callorhinus ursinus) Can Be
Explained by Pollock (Gadus chalcogrammus) Catches in the Eastern Bering Sea. J. Mar. Sci. Eng. 2021, 9, 975. [CrossRef]
89.
Kuhn, C.E.; Baker, J.D.; Towell, R.G.; Ream, R.R. Evidence of localized resource depletion following a natural colonization event
by a large marine predator. J. Anim. Ecol. 2014, 83, 1169–1177. [CrossRef]
90.
Eberhardt, L.L.; Siniff, D.B. Population Dynamics and Marine Mammal Management Policies. J. Fish. Res. Board Can.
1977
, 34,
183–190. [CrossRef]
91. Leighton, F.A. Surveillance of wild animal diseases in Europe. Rev. Sci. Et Tech. Int. Off. Epizoot. 1995, 14, 819–830.
92. Cooper, J.E. Diagnostic pathology of selected diseases in wildlife. Rev. Sci. Tech. 2002, 21, 77–89. [CrossRef]
93.
Küker, S.; Faverjon, C.; Furrer, L.; Berezowski, J.; Posthaus, H.; Rinaldi, F.; Vial, F. The value of necropsy reports for animal health
surveillance. BMC Vet. Res. 2018, 14, 191. [CrossRef]
94.
McAloose, D.; Colegrove, K.M.; Newton, A.L. Chapter 1—Wildlife Necropsy. In Pathology of Wildlife and Zoo Animals; Terio, K.A.,
McAloose, D., Leger, J.S., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 1–20.
95.
Colegrove, K.M.; Burek-Huntington, K.A.; Roe, W.; Siebert, U. Pinnipediae. Pathol. Wildl. Zoo Anim.
2018
, 4, 569–592. [CrossRef]
96.
Yano, K.; Fowler, C. Population Changes in Northern Fur Seal Rookeries at Reef Rookery on St. Paul Island of the Pribilof Islands,
Alaska. Available online: https://apps-afsc.fisheries.noaa.gov/pubs/posters/pdfs/pYano02_n-fur-seal-aerial.pdf (accessed on
15 April 2022).
97.
Yano, K.M.; Tingg, J.; Fowler, C. Northern Fur Seal Rookery Photo Archive: Aerial and Ground-Level Photos, Pribilof Islands, Alaska,
1895–2006; Alaska Fisheries Science Center, NOAA, National Marine Fisheries Service: Seattle, WA, USA, 2009; p. 62.

Oceans 2022, 3 318
98.
Patyk, K.A.; Duncan, C.; Nol, P.; Sonne, C.; Laidre, K.; Obbard, M.; Wiig, Ø.; Aars, J.; Regehr, E.; Gustafson, L.L.; et al. Establishing
a definition of polar bear (Ursus maritimus) health: A guide to research and management activities. Sci. Total Environ.
2015
, 514,
371–378. [CrossRef] [PubMed]
99.
Krause, G. How can infectious diseases be prioritized in public health? A standardized prioritization scheme for discussion.
EMBO Rep. 2008, 9 (Suppl. S1), S22–S27. [CrossRef] [PubMed]
100.
O’Brien, D.; Scudamore, J.; Charlier, J.; Delavergne, M. DISCONTOOLS: A database to identify research gaps on vaccines,
pharmaceuticals and diagnostics for the control of infectious diseases of animals. BMC Vet. Res. 2016, 13, 1. [CrossRef]
101.
Humblet, M.-F.; Vandeputte, S.; Albert, A.; Gosset, C.; Kirschvink, N.; Haubruge, E.; Fecher-Bourgeois, F.; Pastoret, P.-P.;
Saegerman, C. Multidisciplinary and evidence-based method for prioritizing diseases of food-producing animals and zoonoses.
Emerg. Infect. Dis. 2012, 18, e1. [CrossRef]
102.
Cardoen, S.; Van Huffel, X.; Berkvens, D.; Quoilin, S.; Ducoffre, G.; Saegerman, C.; Speybroeck, N.; Imberechts, H.; Herman, L.;
Ducatelle, R.; et al. Evidence-based semiquantitative methodology for prioritization of foodborne zoonoses. Foodborne Pathog.
Dis. 2009, 6, 1083–1096. [CrossRef]
103.
McKenzie, J.; Simpson, H.; Langstaff, I. Development of methodology to prioritise wildlife pathogens for surveillance. Prev. Vet.
Med. 2007, 81, 194–210. [CrossRef]
104.
Ng, V.; Sargeant, J.M. A stakeholder-informed approach to the identification of criteria for the prioritization of zoonoses in
Canada. PLoS ONE 2012, 7, e29752. [CrossRef]
105.
Waltzek, T.B.; Cortés-Hinojosa, G.; Wellehan, J.F.X., Jr.; Gray, G.C. Marine mammal zoonoses: A review of disease manifestations.
Zoonoses Public Health 2012, 59, 521–535. [CrossRef]
106.
Hunt, T.D.; Ziccardi, M.H.; Gulland, F.M.D.; Yochem, P.K.; Hird, D.W.; Rowles, T.; Mazet, J.A.K. Health risks for marine mammal
workers. Dis. Aquat. Org. 2008, 81, 81–92. [CrossRef]
107. Parker, N.R.; Barralet, J.H.; Bell, A.M. Q fever. Lancet 2006, 367, 679–688. [CrossRef]
108.
Kersh, G.J.; Fitzpatrick, K.; Pletnikoff, K.; Brubaker, M.; Bruce, M.; Parkinson, A. Prevalence of serum antibodies to Coxiella
burnetii in Alaska Native Persons from the Pribilof Islands. Zoonoses Public Health 2020, 67, 89–92. [CrossRef] [PubMed]
109.
Dubey, J.P.; Murata, F.H.A.; Cerqueira-Cézar, C.K.; Kwok, O.C.H.; Grigg, M.E. Recent epidemiologic and clinical importance of
Toxoplasma gondii infections in marine mammals: 2009–2020. Vet. Parasitol. 2020, 288, 109296. [CrossRef] [PubMed]
110.
Rea, L.D.; Castellini, J.M.; Avery, J.P.; Fadely, B.S.; Burkanov, V.N.; Rehberg, M.J.; O’Hara, T.M. Regional variations and drivers of
mercury and selenium concentrations in Steller sea lions. Sci. Total Environ. 2020, 744, 140787. [CrossRef]
111.
Gulland, F.M.; Koski, M.; Lowenstine, L.J.; Colagross, A.; Morgan, L.; Spraker, T. Leptospirosis in California sea lions (Zalophus
californianus) stranded along the central California coast, 1981–1994. J. Wildl. Dis. 1996, 32, 572–580. [CrossRef]
112.
Barbieri, M.M.; Kashinsky, L.; Rotstein, D.S.; Colegrove, K.M.; Haman, K.H.; Magargal, S.L.; Sweeny, A.R.; Kaufman, A.C.; Grigg,
M.E.; Littnan, C.L. Protozoal-related mortalities in endangered Hawaiian monk seals Neomonachus schauinslandi. Dis. Aquat.
Org. 2016, 121, 85–95. [CrossRef]
113.
Ng, V.; Sargeant, J.M. A quantitative and novel approach to the prioritization of zoonotic diseases in North America: A public
perspective. PLoS ONE 2012, 7, e48519. [CrossRef]
114.
Veltre, D.W.; Veltre, M.J. The Northern Fur Seal: A Subsistence and Commercial Resource for Aleuts of the Aleutian and Pribilof
Islands, Alaska. Études Inuit Stud. 1987, 11, 51–72.
115.
Ostertag, S.K.; Loseto, L.L.; Snow, K.; Lam, J.; Hynes, K.; Gillman, D.V. “That’s how we know they’re healthy”: The inclusion of
traditional ecological knowledge in beluga health monitoring in the Inuvialuit Settlement Region. Arct. Sci.
2018
, 4, 292–320.
[CrossRef]
116.
Breton-Honeyman, K.; Furgal, C.M.; Hammill, M.O. Systematic Review and Critique of the Contributions of Traditional Ecological
Knowledge of Beluga Whales in the Marine Mammal Literature. Arctic 2016, 69, 37–46. [CrossRef]
117.
Wyllie de Echeverria, V.R.; Thornton, T.F. Using traditional ecological knowledge to understand and adapt to climate and
biodiversity change on the Pacific coast of North America. Ambio 2019, 48, 1447–1469. [CrossRef] [PubMed]
118.
Huntington, H.P.; Braem, N.M.; Brown, C.L.; Hunn, E.; Krieg, T.M.; Lestenkof, P.; Noongwook, G.; Sepez, J.; Sigler, M.F.;
Wiese, F.K.; et al. Local and traditional knowledge regarding the Bering Sea ecosystem: Selected results from five indigenous
communities. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2013, 94, 323–332. [CrossRef]
119.
Peacock, S.J.; Mavrot, F.; Tomaselli, M.; Hanke, A.; Fenton, H.; Nathoo, R.; Aleuy, O.A.; Di Francesco, J.; Aguilar, X.F.; Jutha, N.;
et al. Linking co-monitoring to co-management: Bringing together local, traditional, and scientific knowledge in a wildlife status
assessment framework. Arct. Sci. 2020, 6, 247–266. [CrossRef]
